Question: Problem 4 (Spring 2007) A liquid A (density PA = 0.8 g/cm, molar mass 80 lb.me/1b-mol) is mixed with a liquid B (density pb =
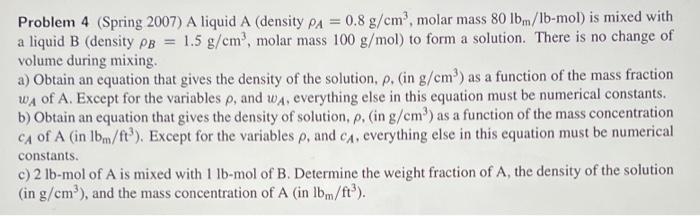
Problem 4 (Spring 2007) A liquid A (density PA = 0.8 g/cm, molar mass 80 lb.me/1b-mol) is mixed with a liquid B (density pb = 1.5 g/cm', molar mass 100 g/mol) to form a solution. There is no change of volume during mixing a) Obtain an equation that gives the density of the solution, p. (in g/cm) as a function of the mass fraction . WA of A. Except for the variables p, and wA, everything else in this equation must be numerical constants. b) Obtain an equation that gives the density of solution, p. (in g/cm') as a function of the mass concentration CA of A (in Ibm/ft?). Except for the variables p, and c. everything else in this equation must be numerical constants. c)2 lb-mol of A is mixed with 1 lb-mol of B. Determine the weight fraction of A, the density of the solution (in g/cm), and the mass concentration of A (in lbm/ft")
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


