Question: Problem 5. The figure below shows a gas stream that contains 18.0 mole percent hexane; the remainder is nitrogen. The stream flows to a condenser,
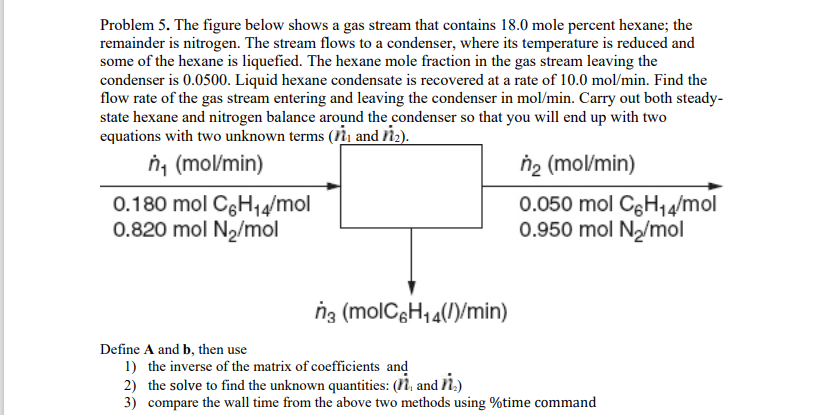
Problem 5. The figure below shows a gas stream that contains 18.0 mole percent hexane; the remainder is nitrogen. The stream flows to a condenser, where its temperature is reduced and some of the hexane is liquefied. The hexane mole fraction in the gas stream leaving the condenser is 0.0500. Liquid hexane condensate is recovered at a rate of 10.0 mol/min. Find the flow rate of the gas stream entering and leaving the condenser in mol/min. Carry out both steady- state hexane and nitrogen balance around the condenser so that you will end up with two equations with two unknown terms (11 and 12). n (mol/min) na (mol/min) 0.180 mol C6H14/mol 0.050 mol C6H14/mol 0.820 mol Ny/mol 0.950 mol Ny/mol ng (molC6H44(1)/min) Define A and b, then use 1) the inverse of the matrix of coefficients and 2) the solve to find the unknown quantities: (17, and ) 3) compare the wall time from the above two methods using %time command
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


