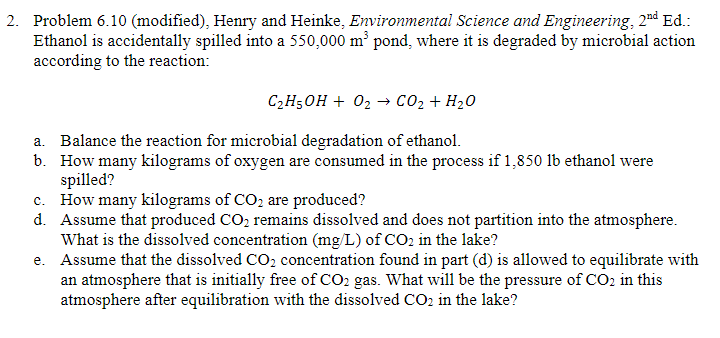
Question: Problem 6 . 1 0 ( modified ) , Henry and Heinke, Environmental Science and Engineering, 2 n d Ed . : Ethanol is accidentally
Problem modified Henry and Heinke, Environmental Science and Engineering, Ed:
Ethanol is accidentally spilled into a pond, where it is degraded by microbial action
according to the reaction:
a Balance the reaction for microbial degradation of ethanol.
b How many kilograms of oxygen are consumed in the process if ethanol were
spilled?
c How many kilograms of are produced?
d Assume that produced remains dissolved and does not partition into the atmosphere.
What is the dissolved concentration of in the lake?
e Assume that the dissolved concentration found in part d is allowed to equilibrate with
an atmosphere that is initially free of gas. What will be the pressure of in this
atmosphere after equilibration with the dissolved in the lake?
Step by Step Solution
There are 3 Steps involved in it
1 Expert Approved Answer
Step: 1 Unlock


Question Has Been Solved by an Expert!
Get step-by-step solutions from verified subject matter experts
Step: 2 Unlock
Step: 3 Unlock


