Question: Problem 6 - Hexagonal Close-Packed System Magnesium and a-Titanium both crystallize in the hexagonal close-packed (hcp) structure of space group. The lattice parameters are: Ti:
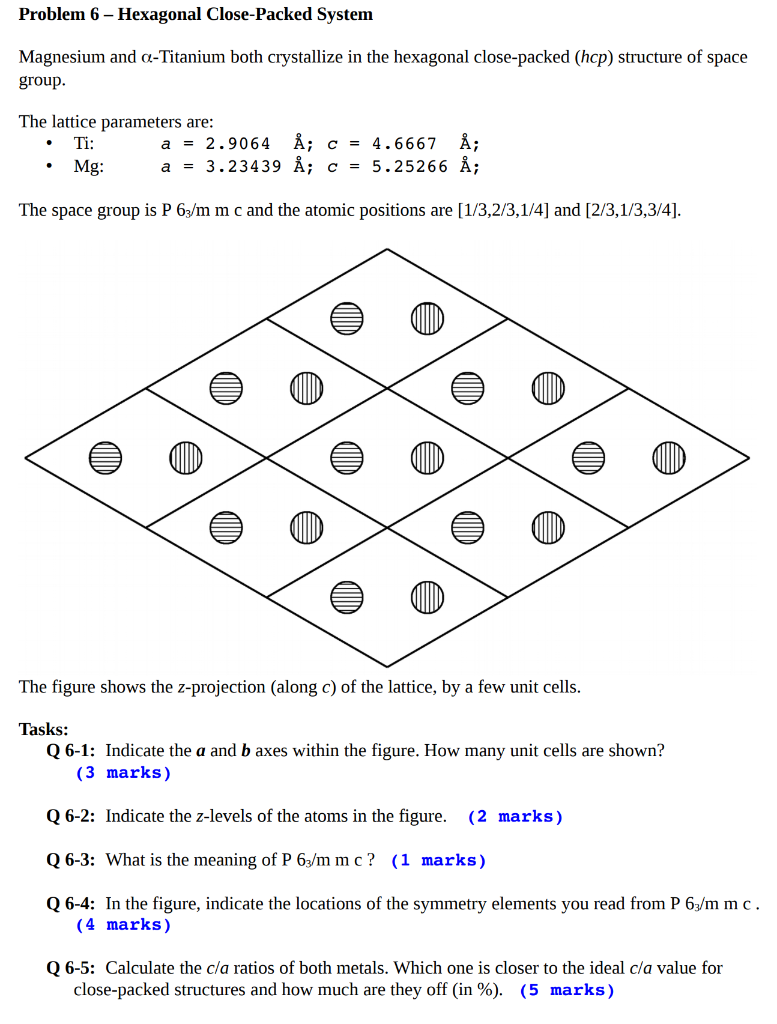
Problem 6 - Hexagonal Close-Packed System Magnesium and a-Titanium both crystallize in the hexagonal close-packed (hcp) structure of space group. The lattice parameters are: Ti: a = 2.9064 ; c = 4.6667 ; Mg: = 3.23439 ; c = 5.25266 ; . a The space group is P63/m m c and the atomic positions are [1/3,2/3,1/4] and [2/3,1/3,3/4]. The figure shows the Z-projection (along C) of the lattice, by a few unit cells. Tasks: Q 6-1: Indicate the a and b axes within the figure. How many unit cells are shown? (3 marks) Q 6-2: Indicate the z-levels of the atoms in the figure. (2 marks) Q 6-3: What is the meaning of P 63/m mc? (1 marks) Q 6-4: In the figure, indicate the locations of the symmetry elements you read from P 63/mmc. (4 marks) Q 6-5: Calculate the cla ratios of both metals. Which one is closer to the ideal cla value for close-packed structures and how much are they off %)
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


