Question: Problem 6. (Internal Energy, Work done, Heat flow in an Ideal Gas Process) P (kPa) A cylinder with a moveable piston contains 0.016 mol of
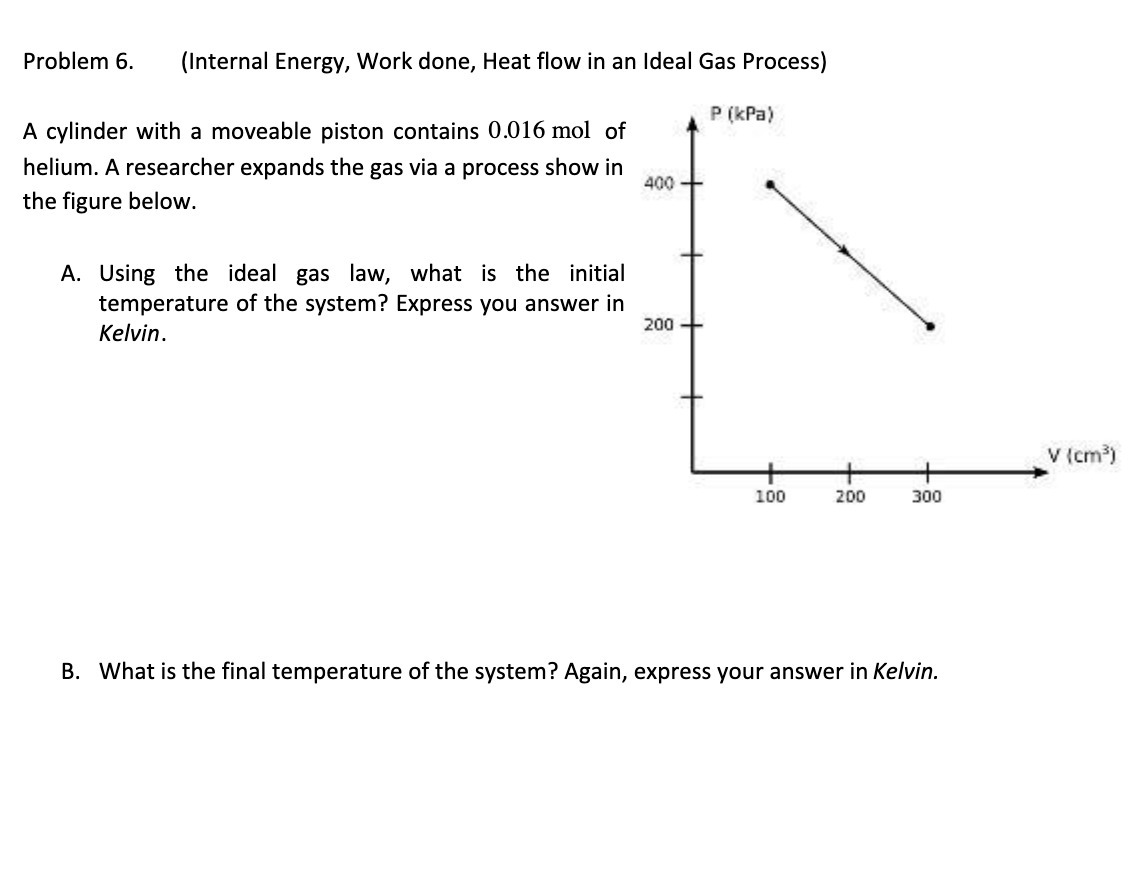
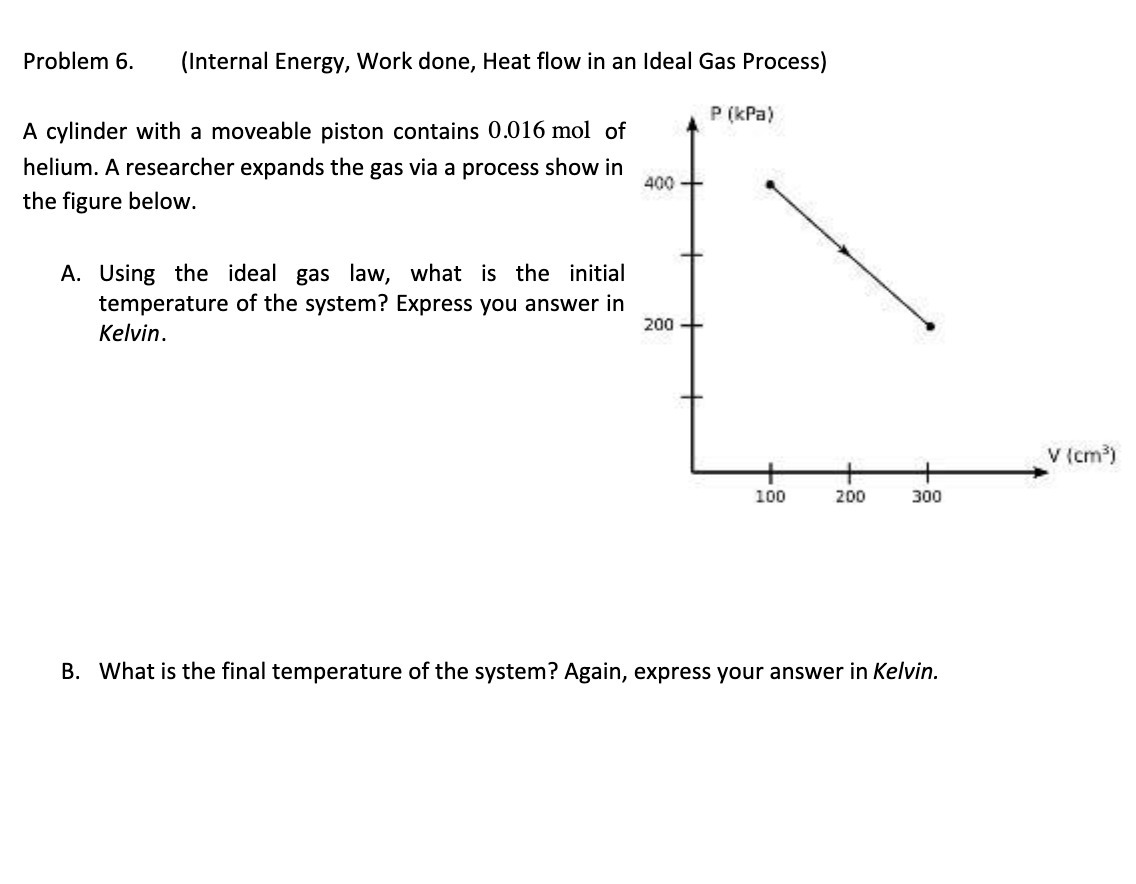
Problem 6. (Internal Energy, Work done, Heat flow in an Ideal Gas Process) P (kPa) A cylinder with a moveable piston contains 0.016 mol of helium. A researcher expands the gas via a process show in 400 - the figure below. A. Using the ideal gas law, what is the initial temperature of the system? Express you answer in Kelvin. 200 - V (cm3) 100 200 300 B. What is the final temperature of the system? Again, express your answer in Kelvin
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


