Question: Problem 8 ( 1 ) Estimate the equilibrium p H of a solution containing 0 . 0 1 M ammonium chloride by using the given
Problem
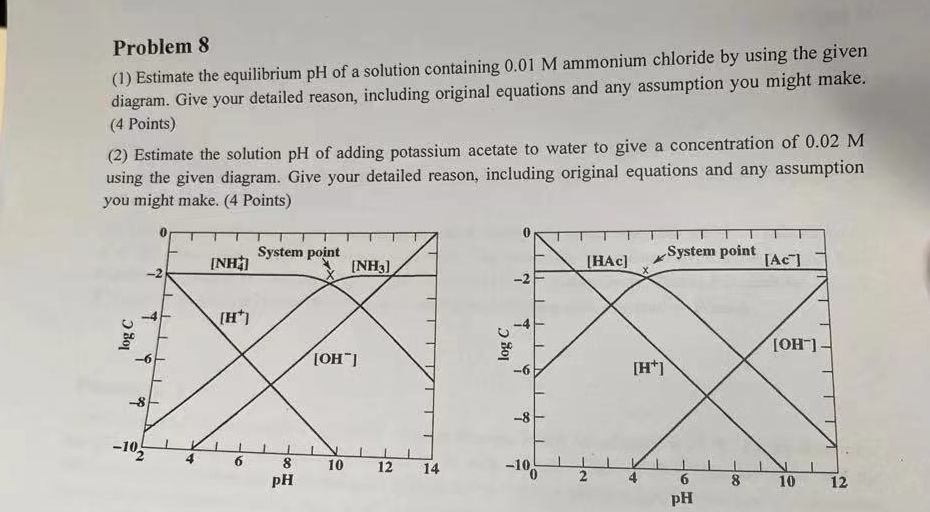
Estimate the equilibrium of a solution containing ammonium chloride by using the given diagram. Give your detailed reason, including original equations and any assumption you might make. Points
Estimate the solution of adding potassium acetate to water to give a concentration of using the given diagram. Give your detailed reason, including original equations and any assumption you might make. Points
Step by Step Solution
There are 3 Steps involved in it
1 Expert Approved Answer
Step: 1 Unlock


Question Has Been Solved by an Expert!
Get step-by-step solutions from verified subject matter experts
Step: 2 Unlock
Step: 3 Unlock


