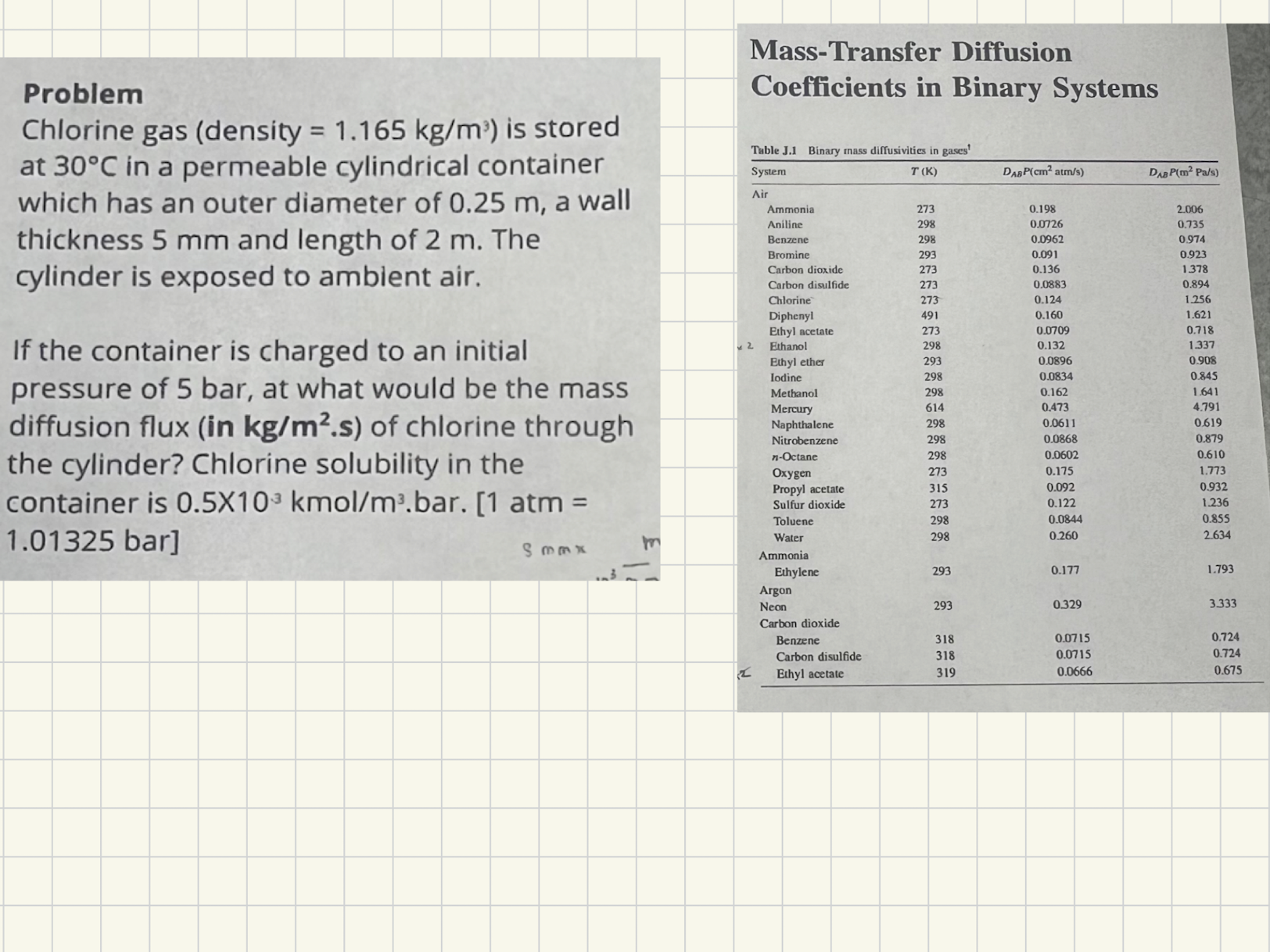
Question: Problem Chlorine gas ( density = 1 . 1 6 5 k g m 3 ) is stored at 3 0 C in a permeable
Problem
Chlorine gas density is stored
at in a permeable cylindrical container
which has an outer diameter of a wall
thickness and length of The
cylinder is exposed to ambient air.
If the container is charged to an initial
pressure of bar at what would be the mass
diffusion flux in of chlorine through
the cylinder? Chlorine solubility in the
container is atm
bar
MassTransfer Diffusion
Coefficients in Binary Systems
Step by Step Solution
There are 3 Steps involved in it
1 Expert Approved Answer
Step: 1 Unlock


Question Has Been Solved by an Expert!
Get step-by-step solutions from verified subject matter experts
Step: 2 Unlock
Step: 3 Unlock


