Question: Problem Statement Consider a liquid phase reaction that can be written as A2B+C. The reaction follows the following second-order rate law: (rA)= kCA2, such that
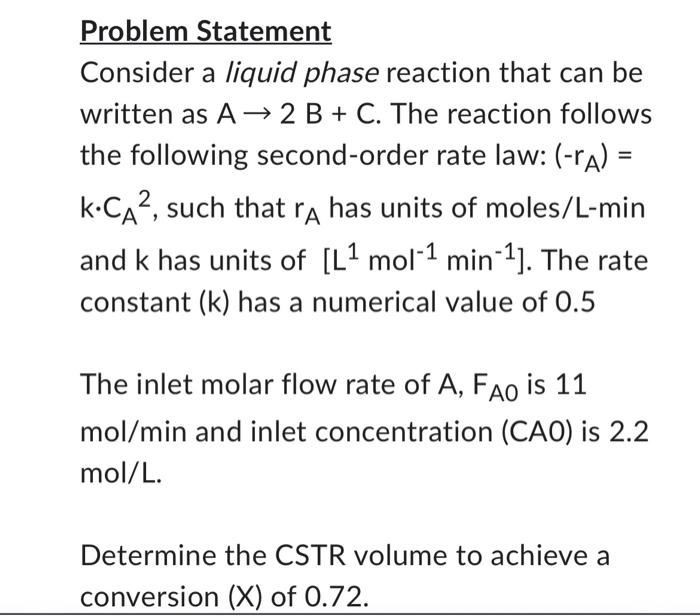
Problem Statement Consider a liquid phase reaction that can be written as A2B+C. The reaction follows the following second-order rate law: (rA)= kCA2, such that rA has units of moles/L-min and k has units of [L1mol1min1]. The rate constant (k ) has a numerical value of 0.5 The inlet molar flow rate of A,FAO is 11 mol/min and inlet concentration (CA0) is 2.2 mol/L. Determine the CSTR volume to achieve a conversion (X) of 0.72
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


