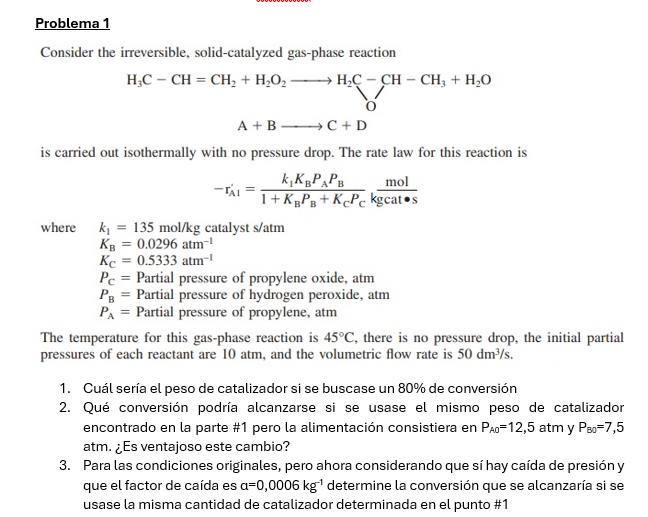
Question: Problema 1 Consider the irreversible, solid - catalyzed gas - phase reaction H 3 C - C H = C H 2 + H 2
Problema
Consider the irreversible, solidcatalyzed gasphase reaction
BlongrightarrowC
is carried out isothermally with no pressure drop. The rate law for this reaction is
where catalyst
Partial pressure of propylene oxide, atm
Partial pressure of hydrogen peroxide, atm
Partial pressure of propylene, atm
The temperature for this gasphase reaction is there is no pressure drop, the initial partial pressures of each reactant are atm, and the volumetric flow rate is
Cul sera el peso de catalizador si se buscase un de conversin
Qu conversin podra alcanzarse si se usase el mismo peso de catalizador encontrado en la parte # pero la alimentacin consistiera en atm y atm. Es ventajoso este cambio?
Para las condiciones originales, pero ahora considerando que s hay cada de presin y que el factor de cada es determine la conversin que se alcanzara si se usase la misma cantidad de catalizador determinada en el punto #
Step by Step Solution
There are 3 Steps involved in it
1 Expert Approved Answer
Step: 1 Unlock


Question Has Been Solved by an Expert!
Get step-by-step solutions from verified subject matter experts
Step: 2 Unlock
Step: 3 Unlock


