Question: 12. Hydrocarbon mixtures are used as fuels. (State the atomic mass of each atom you are using for each solution) How many grams of
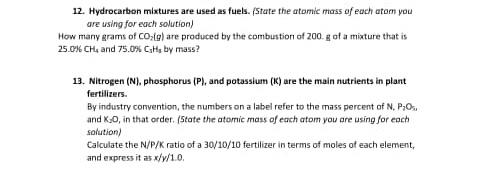
12. Hydrocarbon mixtures are used as fuels. (State the atomic mass of each atom you are using for each solution) How many grams of CO:(g) are produced by the combustion of 200. g of a mixture that is 25.0% CH, and 75.0% Catt, by mass? 13. Nitrogen (N), phosphorus (P), and potassium (K) are the main nutrients in plant fertilizers. By industry convention, the numbers on a label refer to the mass percent of N, P;O, and K0, in that order. (State the atomic mass of each atom you are using for each solution) Calculate the N/P/K ratio of a 30/10/10 fertilizer in terms of moles of each element, and express it as x/y/1.0.
Step by Step Solution
3.42 Rating (149 Votes )
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


