Question: Propane has __ sigma (single) bonds, and __ single lines in its Lewis structure. please answer questions 7-13 Propane has sigma (single) bonds, and single
Propane has __ sigma (single) bonds, and __ single lines in its Lewis structure.
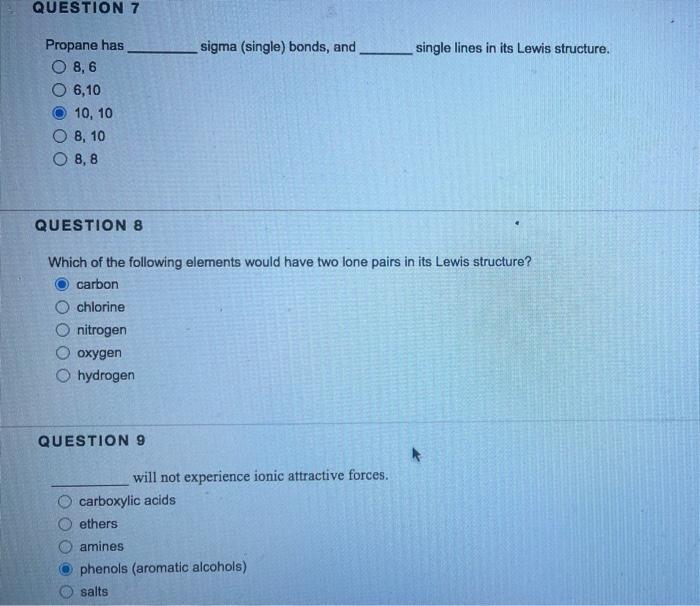
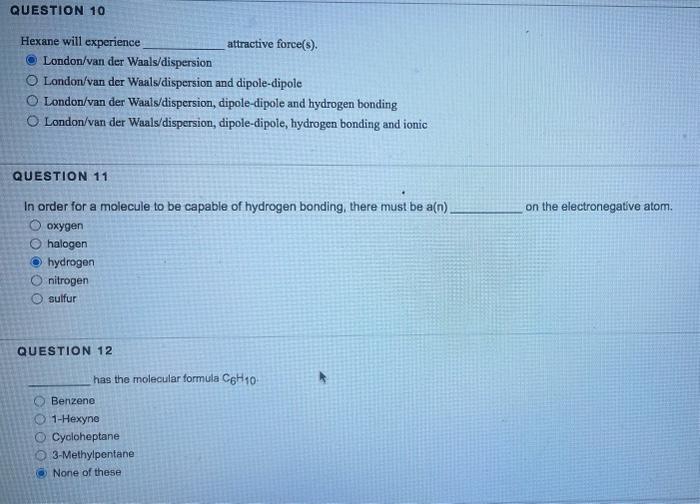
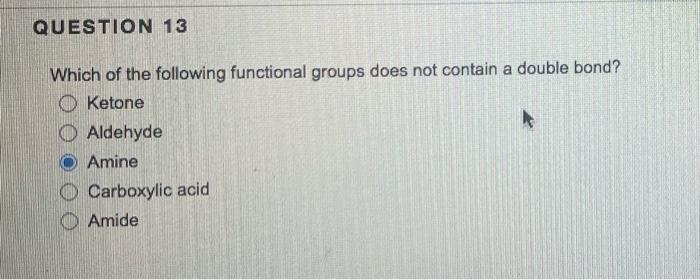
Propane has sigma (single) bonds, and single lines in its Lewis structure. 8,6 6,10 10,10 8,10 8,8 QUESTION 8 Which of the following elements would have two lone pairs in its Lewis structure? carbon chlorine nitrogen oxygen hydrogen QUESTION 9 will not experience ionic attractive forces. carboxylic acids ethers amines phenols (aromatic alcohols) salts Hexane will experience attractive force(s). London/van der Waals/dispersion London/van der Waals/dispersion and dipole-dipole London/van der Waals/dispersion, dipole-dipole and hydrogen bonding London/van der Waals/dispersion, dipole-dipole, hydrogen bonding and ionic QUESTION 11 In order for a molecule to be capable of hydrogen bonding, there must be a(n) on the electronegative atom. oxygen halogen hydrogen nitrogen sulfur QUESTION 12 has the molecular tormula C6H10. Benzene 1-Hexyne Cycloheptane 3-Methylpentane None of these Which of the following functional groups does not contain a double bond? Ketone Aldehyde Amine Carboxylic acid Amide
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


