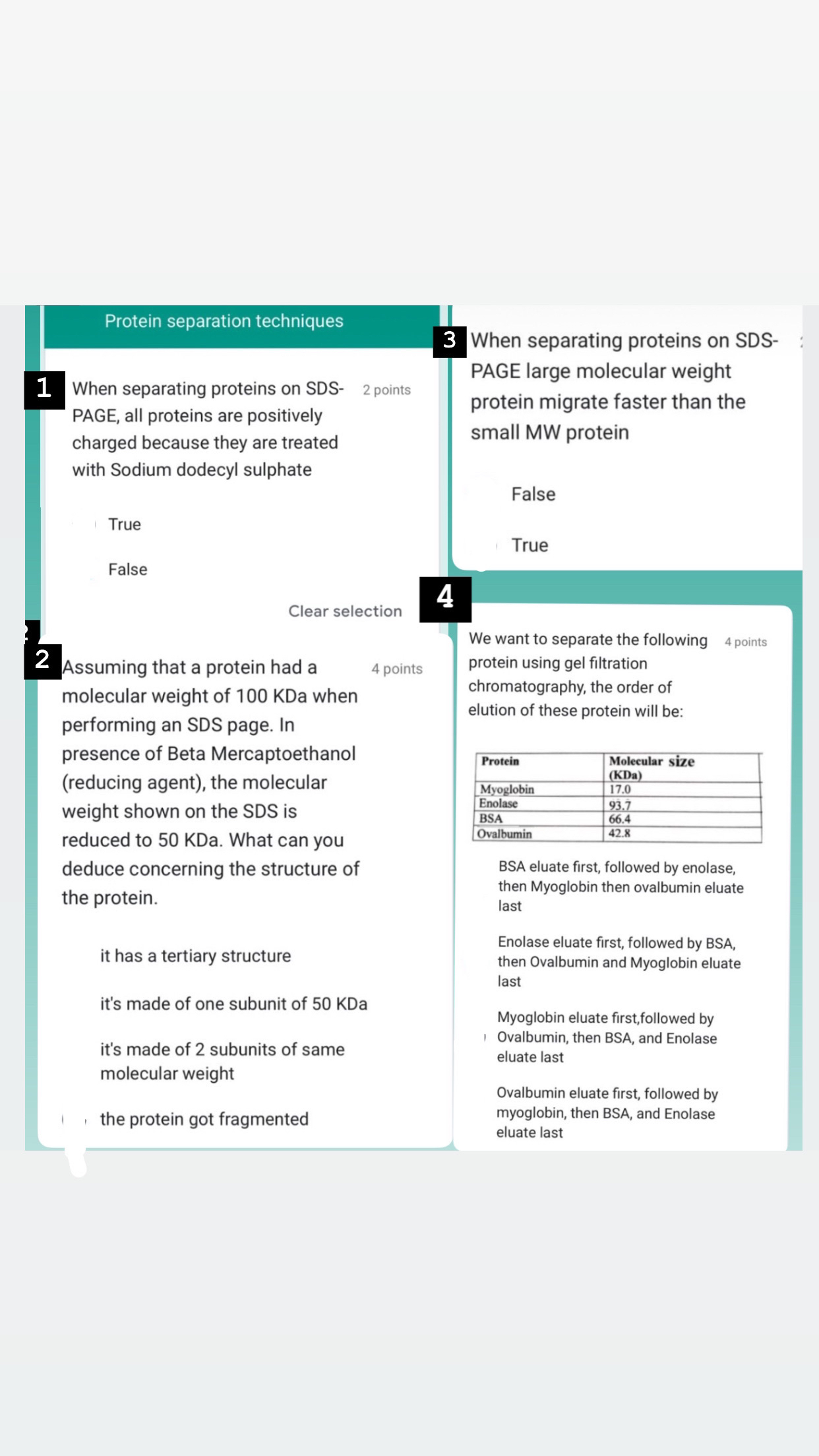
Question: Protein separation techniques 1 When separating proteins on SDS - 2 points PAGE, all proteins are positively charged because they are treated with Sodium dodecyl
Protein separation techniques
When separating proteins on SDS
points PAGE, all proteins are positively charged because they are treated with Sodium dodecyl sulphate
True
False
Clear selection
Assuming that a protein had a
points
molecular weight of KDa when performing an SDS page. In presence of Beta Mercaptoethanol reducing agent the molecular weight shown on the SDS is reduced to KDa What can you deduce concerning the structure of the protein.
it has a tertiary structure
it's made of one subunit of KDa
it's made of subunits of same molecular weight
the protein got fragmented
When separating proteins on SDSPAGE large molecular weight protein migrate faster than the small MW protein
False
True
We want to separate the following
points protein using gel filtration chromatography, the order of elution of these protein will be:
tableProteintableMolecular sizeKDaMyoglobinEnolaseBSAOvalbumin
BSA eluate first, followed by enolase, then Myoglobin then ovalbumin eluate last
Enolase eluate first, followed by BSA, then Ovalbumin and Myoglobin eluate last
Myoglobin eluate first,followed by Ovalbumin, then BSA, and Enolase eluate last
Ovalbumin eluate first, followed by myoglobin, then BSA, and Enolase eluate last
The course is biochemistry
Step by Step Solution
There are 3 Steps involved in it
1 Expert Approved Answer
Step: 1 Unlock


Question Has Been Solved by an Expert!
Get step-by-step solutions from verified subject matter experts
Step: 2 Unlock
Step: 3 Unlock


