Question: Pure iron goes through a polymorphic change from BCC to FCC upon heating through 912C. Calculate the volume change associated with the change in
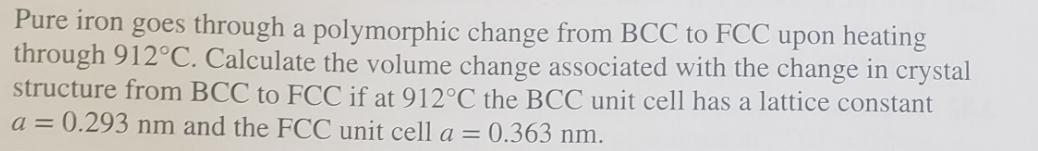
Pure iron goes through a polymorphic change from BCC to FCC upon heating through 912C. Calculate the volume change associated with the change in crystal structure from BCC to FCC if at 912C the BCC unit cell has a lattice constant a = 0.293 nm and the FCC unit cell a = 0.363 nm.
Step by Step Solution
There are 3 Steps involved in it
To calculate the volume change associated with the change in crystal structure from bodycentered cub... View full answer

Get step-by-step solutions from verified subject matter experts


