Question: PYTHON PROGRAMMING LANGUAGE 1: Nernst Equation (17) The Nernst Equation is a famous equation in chemistry that finds the reduction potential (E) of a chemical
PYTHON PROGRAMMING LANGUAGE
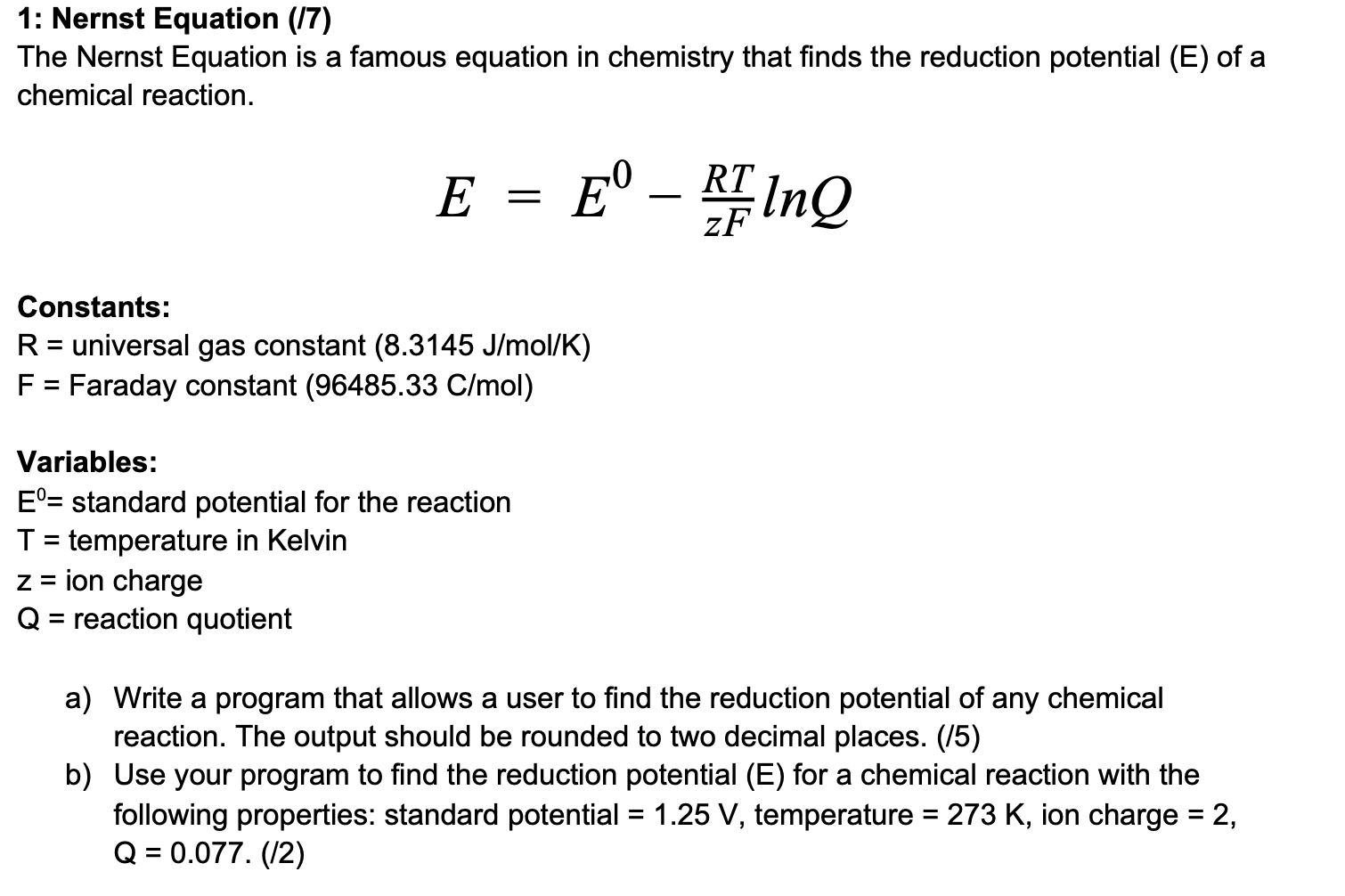
1: Nernst Equation (17) The Nernst Equation is a famous equation in chemistry that finds the reduction potential (E) of a chemical reaction. E = E RF Ing Constants: R = universal gas constant (8.3145 J/mol/K) F = Faraday constant (96485.33 C/mol) Variables: E= standard potential for the reaction T = temperature in Kelvin z = ion charge Q = reaction quotient a) Write a program that allows a user to find the reduction potential of any chemical reaction. The output should be rounded to two decimal places. (15) b) Use your program to find the reduction potential (E) for a chemical reaction with the following properties: standard potential = 1.25 V, temperature = 273 K, ion charge = 2, Q = 0.077. (12)
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


