Question: Python Question: Thanks. Appreciate it. Using the following atomic weights carbon hydrogen oxygen 12.0110 1.0079 15.9994 compute the molecular weight of a compound of carbon,
Python Question:
Thanks. 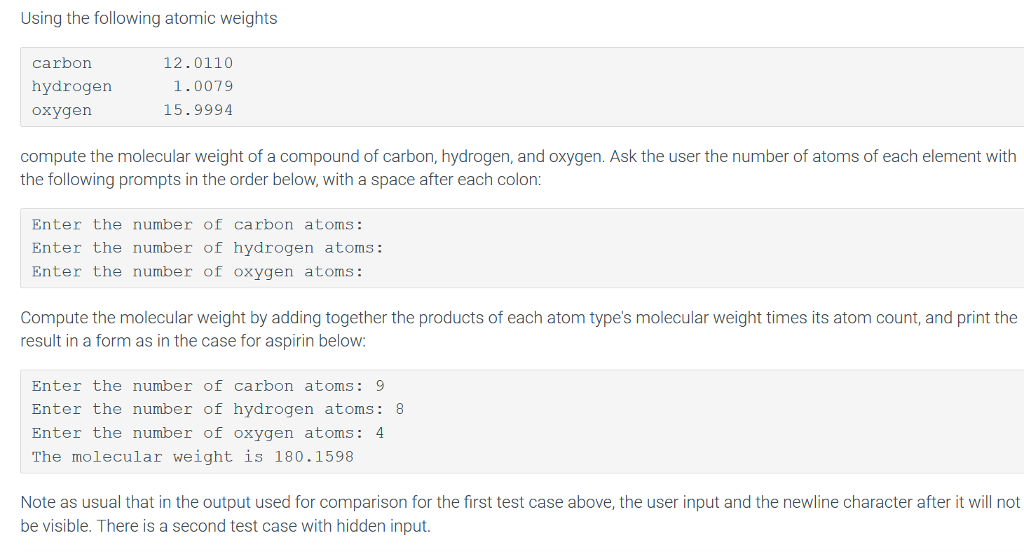 Appreciate it.
Appreciate it.
Using the following atomic weights carbon hydrogen oxygen 12.0110 1.0079 15.9994 compute the molecular weight of a compound of carbon, hydrogen, and oxygen. Ask the user the number of atoms of each element with the following prompts in the order below, with a space after each colon: Enter the number of carbon atoms: Enter the number of hydrogen atoms: Enter the number of oxygen atoms: Compute the molecular weight by adding together the products of each atom type's molecular weight times its atom count, and print the result in a form as in the case for aspirin below: Enter the number of carbon atoms: 9 Enter the number of hydrogen atoms: 8 Enter the number of oxygen atoms: 4 The molecular weight is 180.1598 Note as usual that in the output used for comparison for the first test case above, the user input and the newline character after it will not be visible. There is a second test case with hidden input
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


