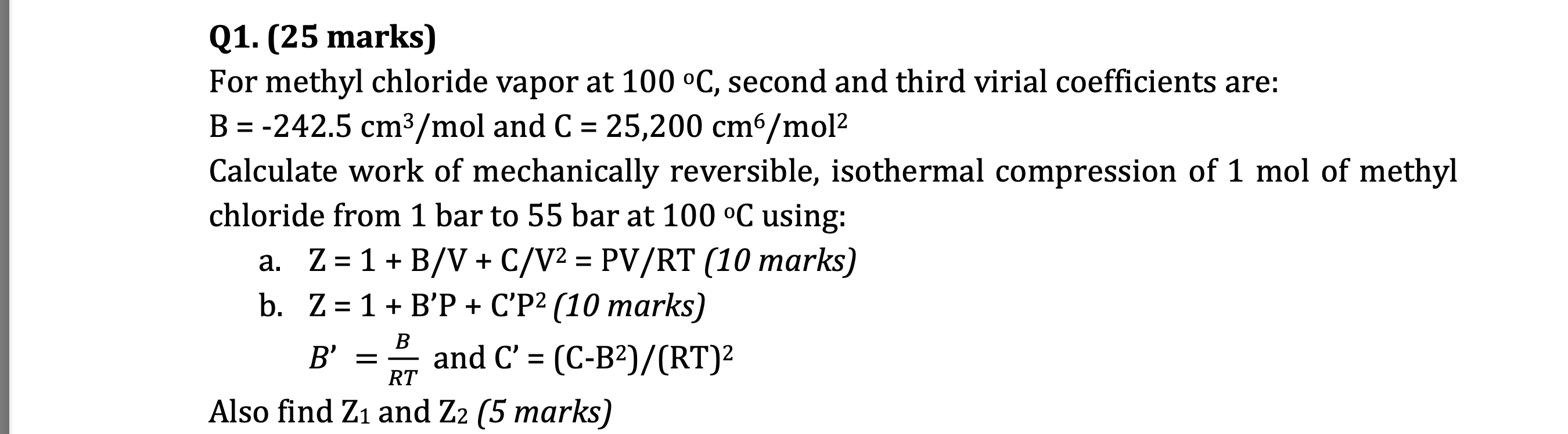
Question: Q 1 . ( 2 5 marks ) For methyl chloride vapor at 1 0 0 C , second and third virial coefficients are: B
Q marks
For methyl chloride vapor at second and third virial coefficients are:
and
Calculate work of mechanically reversible, isothermal compression of mol of methyl
chloride from bar to bar at using:
a marks
b marks
and
Also find and marksPLEASE ACCOUNT FOR ALL CONVERSIONS PLEASE IT CONFUSES ME EXAIVIPLE PRUBLEIVI For methyl chloride at oC the second and third virial coefficient are:
Bcmmol Ccmmol
Calculate the work of mechanically reversible, isothermal compression of mol of methyl chloride from bar to bar at oC Base calculations on the following forms of the virial equation: a ZBVCVb ZB P P where BBR T and C CBR T Why don't both equations give the same result?
Step by Step Solution
There are 3 Steps involved in it
1 Expert Approved Answer
Step: 1 Unlock


Question Has Been Solved by an Expert!
Get step-by-step solutions from verified subject matter experts
Step: 2 Unlock
Step: 3 Unlock


