Question: Q 1 . ( 3 0 marks ) The P - x - y diagram of C H C l 3 ( component - 1
Q marks
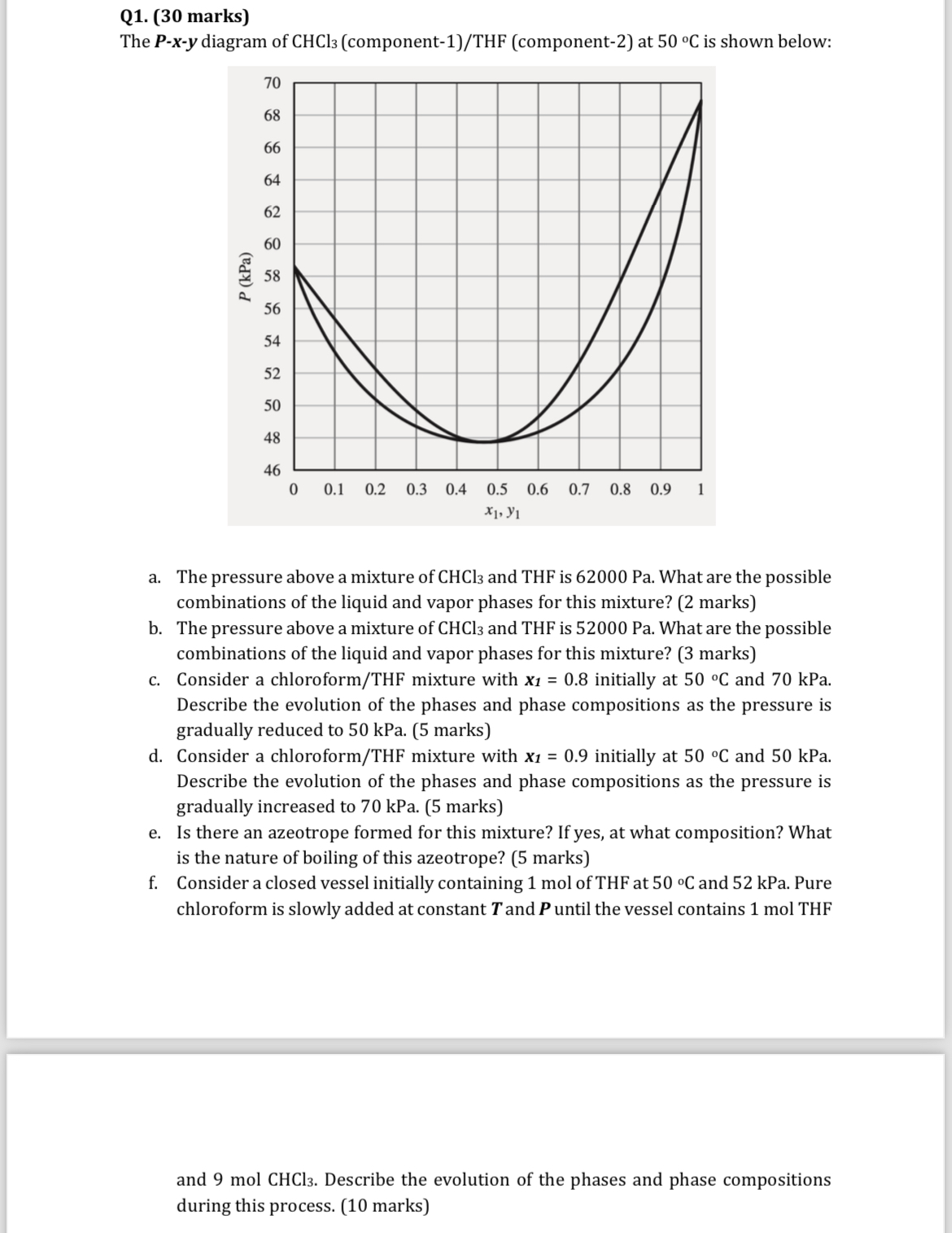
The diagram of componentTHF component at is shown below:
a The pressure above a mixture of and is What are the possible combinations of the liquid and vapor phases for this mixture? marks
b The pressure above a mixture of and is What are the possible combinations of the liquid and vapor phases for this mixture? marks
c Consider a chloroformTHF mixture with initially at and kPa. Describe the evolution of the phases and phase compositions as the pressure is gradually reduced to kPa. marks
d Consider a chloroformTHF mixture with initially at and kPa. Describe the evolution of the phases and phase compositions as the pressure is gradually increased to kPa. marks
e Is there an azeotrope formed for this mixture? If yes, at what composition? What is the nature of boiling of this azeotrope? marks
f Consider a closed vessel initially containing mol of THF at and kPa. Pure chloroform is slowly added at constant and until the vessel contains mol THF
and Describe the evolution of the phases and phase compositions during this process. marks
Step by Step Solution
There are 3 Steps involved in it
1 Expert Approved Answer
Step: 1 Unlock


Question Has Been Solved by an Expert!
Get step-by-step solutions from verified subject matter experts
Step: 2 Unlock
Step: 3 Unlock


