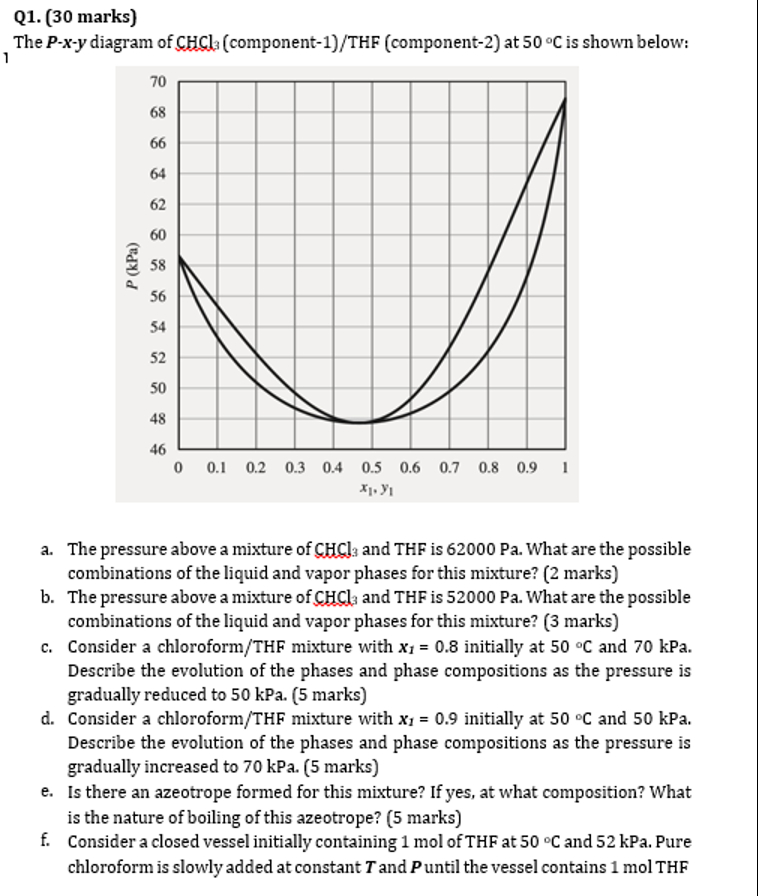
Question: Q 1 . ( 3 0 marks ) The P - x - y diagram of C H C l 3 ( component - 1
Q marks
The diagram of componentTHF component at is shown below:
a The pressure above a mixture of and is What are the possible
combinations of the liquid and vapor phases for this mixture? marks
b The pressure above a mixture of and THF is What are the possible
combinations of the liquid and vapor phases for this mixture? marks
c Consider a chloroformTHF mixture with initially at and kPa.
Describe the evolution of the phases and phase compositions as the pressure is
gradually reduced to kPa. marks
d Consider a chloroformTHF mixture with initially at and kPa.
Describe the evolution of the phases and phase compositions as the pressure is
gradually increased to kPa. marks
e Is there an azeotrope formed for this mixture? If yes, at what composition? What
is the nature of boiling of this azeotrope? marks
f Consider a closed vessel initially containing mol of THF at and kPa. Pure
chloroform is slowly added at constant and until the vessel contains mol THF
I want the answer for e and f only
Step by Step Solution
There are 3 Steps involved in it
1 Expert Approved Answer
Step: 1 Unlock


Question Has Been Solved by an Expert!
Get step-by-step solutions from verified subject matter experts
Step: 2 Unlock
Step: 3 Unlock


