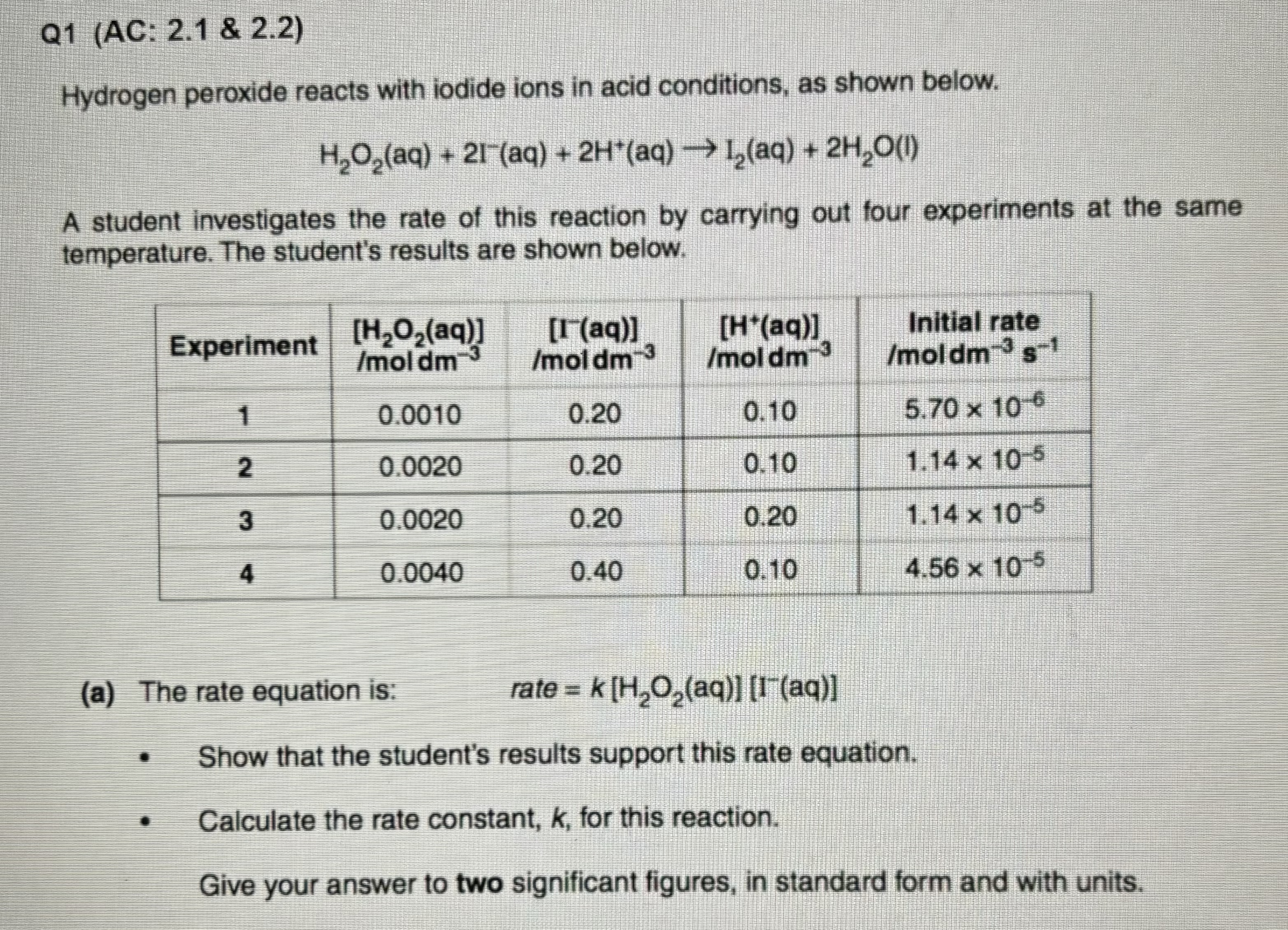
Question: Q 1 ( AC: 2 . 1 & 2 . 2 ) Hydrogen peroxide reacts with iodide ions in acid conditions, as shown below. H
QAC: &
Hydrogen peroxide reacts with iodide ions in acid conditions, as shown below.
A student investigates the rate of this reaction by carrying out four experiments at the same
temperature. The student's results are shown below.
a The rate equation is: rate
Show that the student's results support this rate equation.
Calculate the rate constant, for this reaction.
Give your answer to two significant figures, in standard form and with units.
Step by Step Solution
There are 3 Steps involved in it
1 Expert Approved Answer
Step: 1 Unlock


Question Has Been Solved by an Expert!
Get step-by-step solutions from verified subject matter experts
Step: 2 Unlock
Step: 3 Unlock


