Question: Q 2 . a ) Prove that: P V c T c = 3 8 R ? Find the critical temperature and critical pressure of
Q a Prove that: Find the critical temperature and critical pressure of the gas?
Qa Starting from the main KMT equation show that
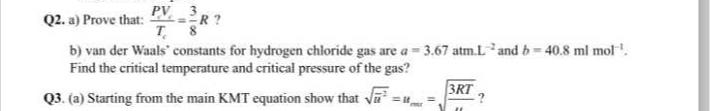
Step by Step Solution
There are 3 Steps involved in it
1 Expert Approved Answer
Step: 1 Unlock


Question Has Been Solved by an Expert!
Get step-by-step solutions from verified subject matter experts
Step: 2 Unlock
Step: 3 Unlock


