Question: Q # 2. An endothermic, reversible isomerization reaction (A + B) is carried out in a CSTR, operating at 330 K. The feed consisting of
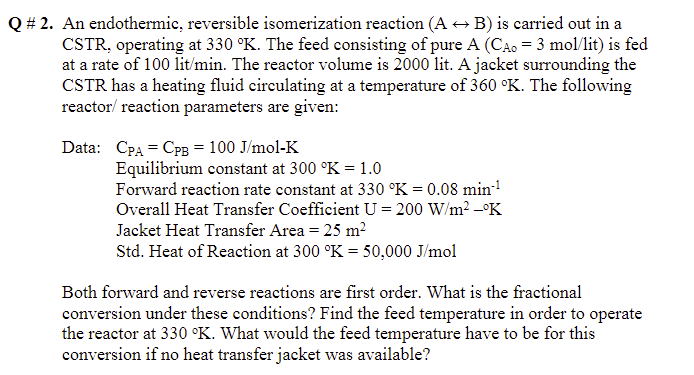
Q # 2. An endothermic, reversible isomerization reaction (A + B) is carried out in a CSTR, operating at 330 K. The feed consisting of pure A (CA. = 3 mol/lit) is fed at a rate of 100 lit/min. The reactor volume is 2000 lit. A jacket surrounding the CSTR has a heating fluid circulating at a temperature of 360 K. The following reactor/ reaction parameters are given: Data: CpA = CpB = 100 J/mol-K Equilibrium constant at 300 K = 1.0 Forward reaction rate constant at 330 K = 0.08 min-1 Overall Heat Transfer Coefficient U= 200 W/m2K Jacket Heat Transfer Area = 25 m2 Std. Heat of Reaction at 300 K = 50,000 J/mol Both forward and reverse reactions are first order. What is the fractional conversion under these conditions? Find the feed temperature in order to operate the reactor at 330 K. What would the feed temperature have to be for this conversion if no heat transfer jacket was available
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


