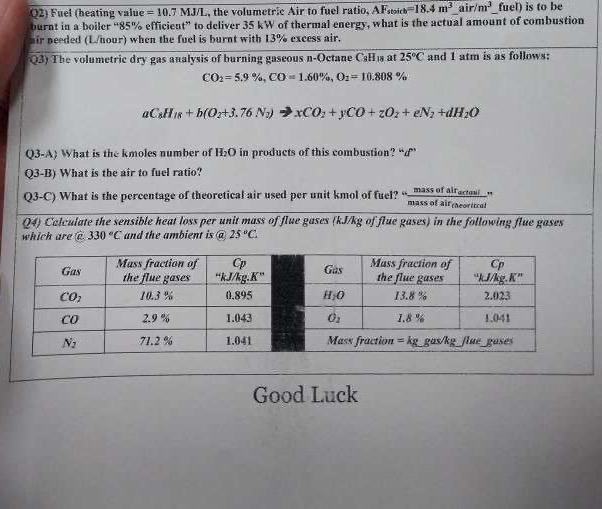
Question: Q 2 ) Fuel ( heating value = 1 0 . 7 M J ? , the volumetric Air to fuel ratio, A F s
Q Fuel heating value the volumetric Air to fuel ratio, air fuel is to be burnt in a boiler efficient" to deliver of thermal energy, what is the actual amount of combustion fir peeded Lnour when the fuel is burat with excess air.
Q The volumetric dry gas analysis of burning gaseous ctane at and atm is as follows:
yCO
QA What is the kmoles number of in products of this combustion"
QB What is the air to fuel ratio?
QC What is the percentage of theoretical air used per unit kmol of ficel? "mass of alf grtoul," which are@ and the ambient is
tableGastableMass fraction ofthe flue gavestable
Step by Step Solution
There are 3 Steps involved in it
1 Expert Approved Answer
Step: 1 Unlock


Question Has Been Solved by an Expert!
Get step-by-step solutions from verified subject matter experts
Step: 2 Unlock
Step: 3 Unlock


