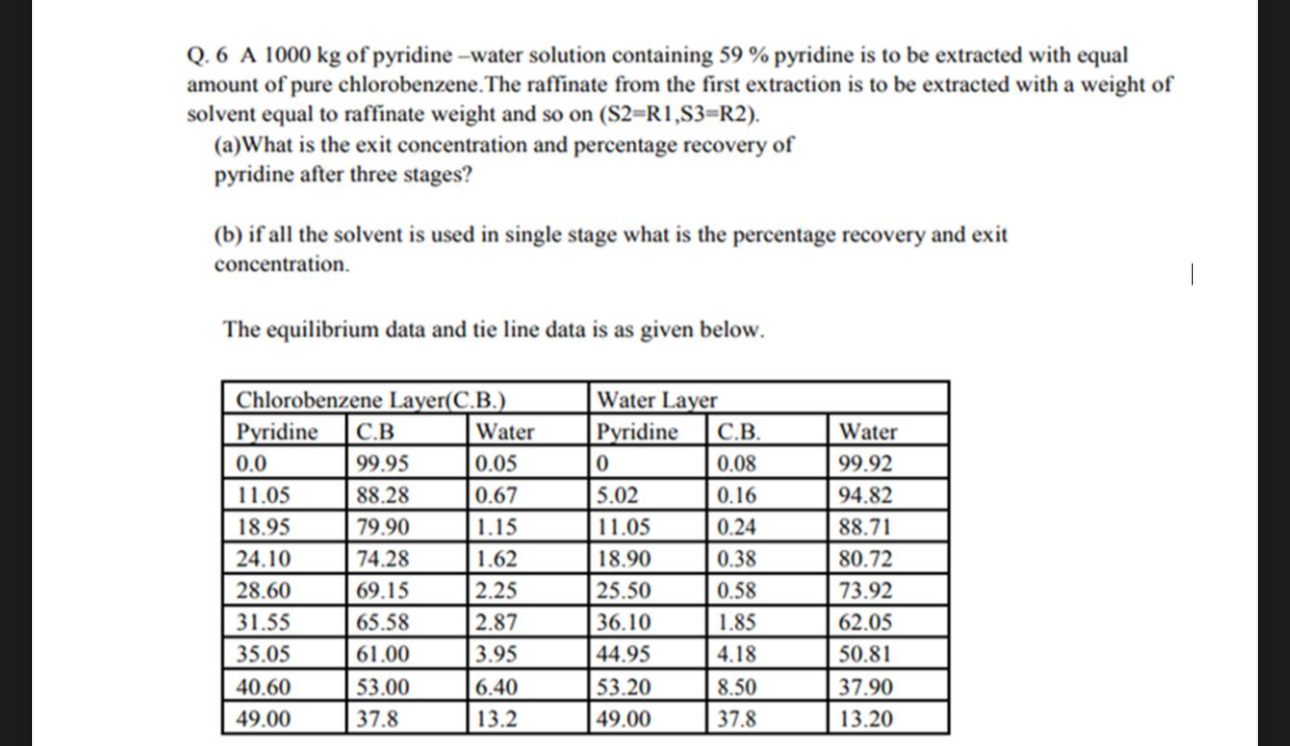
Question: Q . 6 A 1 0 0 0 k g of pyridine - water solution containing 5 9 % pyridine is to be extracted with
Q A of pyridine water solution containing pyridine is to be extracted with equal amount of pure chlorobenzene. The raffinate from the first extraction is to be extracted with a weight of solvent equal to raffinate weight and so on
aWhat is the exit concentration and percentage recovery of pyridine after three stages?
b if all the solvent is used in single stage what is the percentage recovery and exit concentration.
The equilibrium data and tie line data is as given below.
tableChlorobenzene LayerCBWater LayerPyridineCBWater,Pyridine,CBWater,,,
Step by Step Solution
There are 3 Steps involved in it
1 Expert Approved Answer
Step: 1 Unlock


Question Has Been Solved by an Expert!
Get step-by-step solutions from verified subject matter experts
Step: 2 Unlock
Step: 3 Unlock


