Question: Q: The rate constants at the same temperature for the anation of [Cr(H2O)6]3+ with a series of four anions are shown below. a) Are these
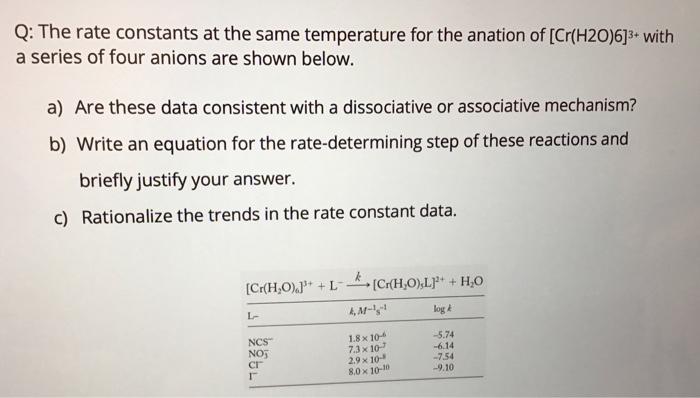
Q: The rate constants at the same temperature for the anation of [Cr(H2O)6]3+ with a series of four anions are shown below. a) Are these data consistent with a dissociative or associative mechanism? b) Write an equation for the rate-determining step of these reactions and briefly justify your answer. c) Rationalize the trends in the rate constant data
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


