Question: Q1 [20 marks) Ethylene can be produced from ethane by dehydrogenation reaction. However, two undesired side reactions will occur simultaneously. Below are all reactions involved
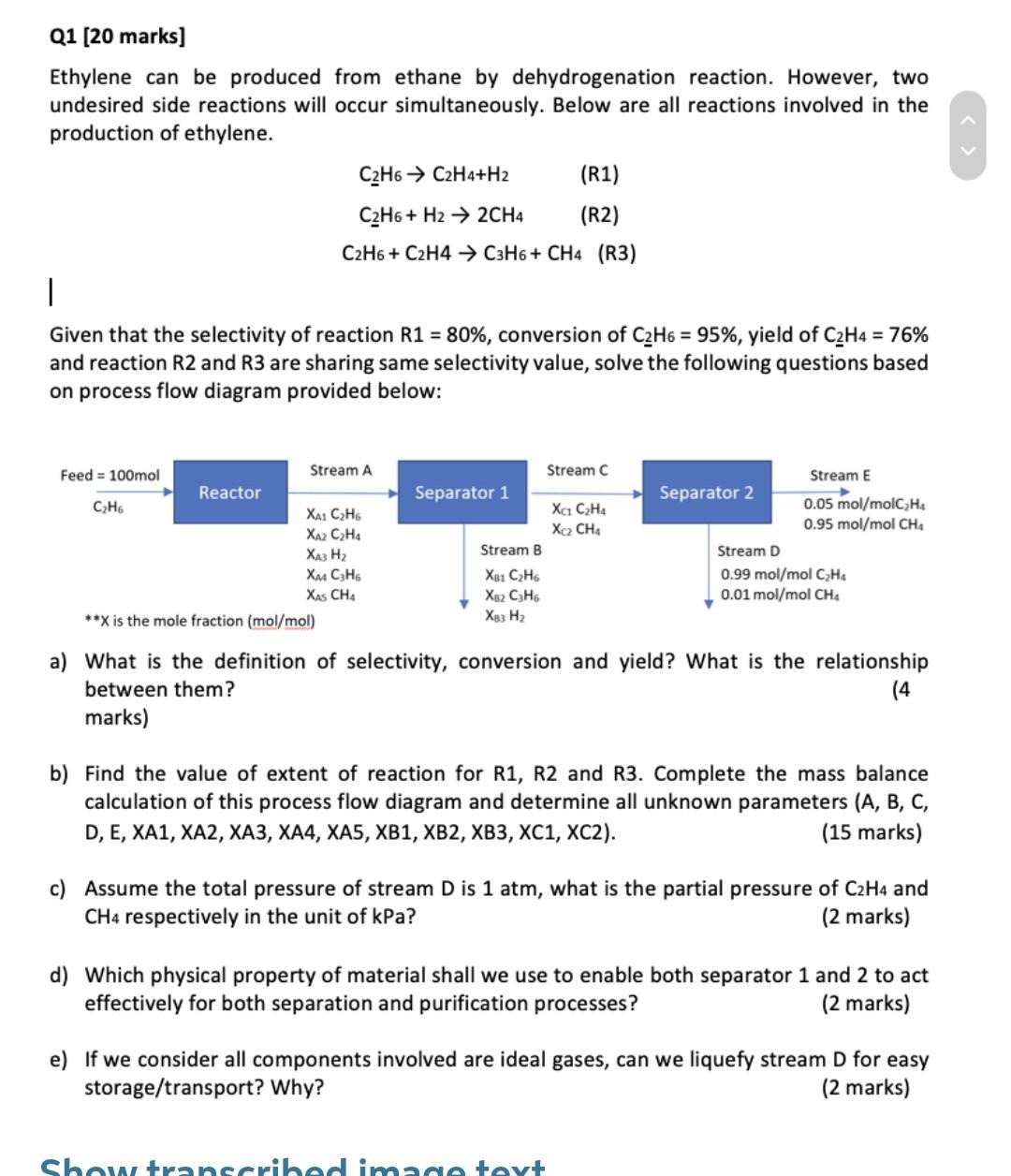
Q1 [20 marks) Ethylene can be produced from ethane by dehydrogenation reaction. However, two undesired side reactions will occur simultaneously. Below are all reactions involved in the production of ethylene. C2H6 C2H4+H2 (R1) C2H6+ H2 2CH4 (R2) C2H6 + C2H4 C3H6+ CH4 (R3) | Given that the selectivity of reaction R1 = 80%, conversion of C2H6 = 95%, yield of C2H4 = 76% and reaction R2 and R3 are sharing same selectivity value, solve the following questions based on process flow diagram provided below: Stream C Separator 1 ct C2H4 Feed = 100mol Stream A Reactor CzH6 XA1 C2H6 XA2 CHA , Xu C3H6 XAS CHA **X is the mole fraction (mol/mol) Stream E Separator 2 0.05 mol/molC Ho 0.95 mol/mol CH4 Stream D 0.99 mol/mol C2H4 0.01mol/mol CH4 Stream B XB1 CH Xx2 C3H6 X83 H2 a) What is the definition of selectivity, conversion and yield? What is the relationship between them? (4 marks) b) Find the value of extent of reaction for R1, R2 and R3. Complete the mass balance calculation of this process flow diagram and determine all unknown parameters (A, B, C, D, E, XA1, XA2, XA3, XA4, XA5, XB1, XB2, XB3, XC1, XC2). (15 marks) c) Assume the total pressure of stream D is 1 atm, what is the partial pressure of C2H4 and CH4 respectively in the unit of kPa? (2 marks) d) Which physical property of material shall we use to enable both separator 1 and 2 to act effectively for both separation and purification processes? (2 marks) e) If we consider all components involved are ideal gases, can we liquefy stream D for easy storage/transport? Why? (2 marks) Showtranscribed image tayt
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


