Question: Q1 (82 points) A saturated liquid feed (600 kg/h) containing 30 wt% ethanol and 70 wt% water is to be separated in a distillation column


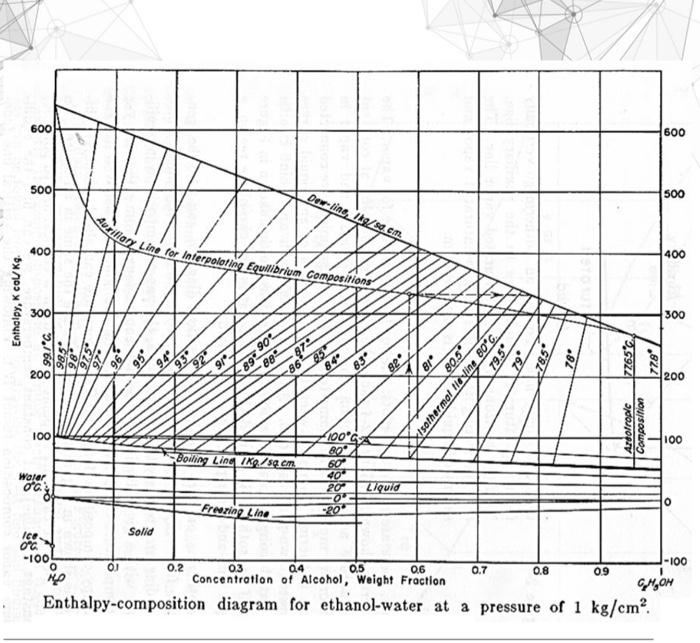
Q1 (82 points) A saturated liquid feed (600 kg/h) containing 30 wt% ethanol and 70 wt% water is to be separated in a distillation column at 101.3 kPa. The bottoms product contains 0.05 wt% ethanol. The vapor distillate from a partial condenser is 85 wt% ethanol. Reflux is a saturated liquid, and the reflux ratio (L/D) is 3 (by mass). Note: 1. Use the given enthalpy-composition diagram to find compositions, if needed. Clearly indicate the data found inside the diagram. 2. Use the given enthalpy-composition diagram to find enthalpies, if needed. Clearly indicate the data found inside the diagram. 3. Use the given enthalpy-composition diagram to find compositions, if needed. Clearly indicate the data found inside the diagram. 4. If a tie line is required, clearly show that using the auxiliary line inside the given enthalpy- composition diagram. 3. Calculate the heat duty of the reboiler. (10 points) 4. What is the expected temperature of the partial condenser? (10 points) 600 600 500! Dewline, / sem 500 Auriliary Line for Interpoloting Equilibrium Compositions 400 400 Enthalpy, K Col kg. 300 300 99/C 985 .86 90 88 97 85 26 83 79.5 .18 8P 785 78 27696 778 2,822 899 84" 200 86 200 95 94 93 80.5 Isothermal ile yine 80*C. 100 Composition Ardotropic 100 Boiling Ling IKQ/s.cn Walar 100 800 60 404 201 0 20 808 Liquid Freezing Line Solid Ice. 0C -100 o -100 01 0.2 04 0.5 0.6 0.7 0.8 09 0.3 Concentration of Alcohol, Weight Fraction Enthalpy-composition diagram for ethanol-water at a pressure of 1 kg/cm GHOH
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


