Question: (Q1) Consider hypothetical platinum carbide, PtC, a solid-state NaCl-type compound with a cubic crystal structure. The unit cell is shown below, with platinum and carbon
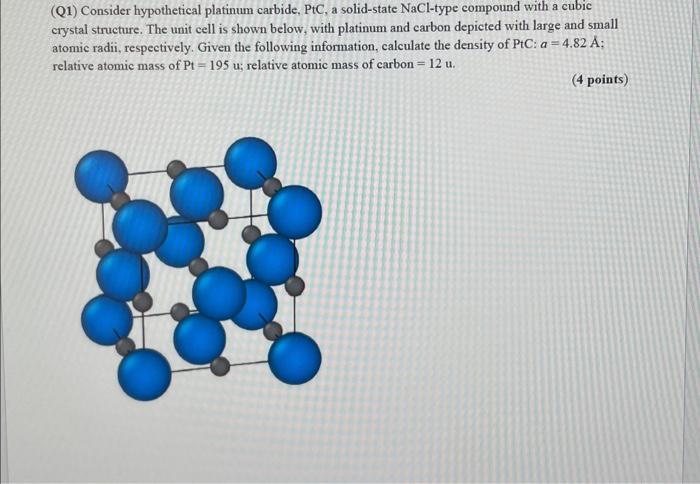
(Q1) Consider hypothetical platinum carbide, PtC, a solid-state NaCl-type compound with a cubic crystal structure. The unit cell is shown below, with platinum and carbon depicted with large and small atomic radii, respectively. Given the following information, calculate the density of PtC: a=4.82A; relative atomic mass of Pt=195u; relative atomic mass of carbon =12u. (4 points)
Step by Step Solution
There are 3 Steps involved in it
1 Expert Approved Answer
Step: 1 Unlock


Question Has Been Solved by an Expert!
Get step-by-step solutions from verified subject matter experts
Step: 2 Unlock
Step: 3 Unlock


