Question: Q1 - Reaction Order Repeat the example we did in class to determine the reaction order, but for additional values of initial concentration Consider a
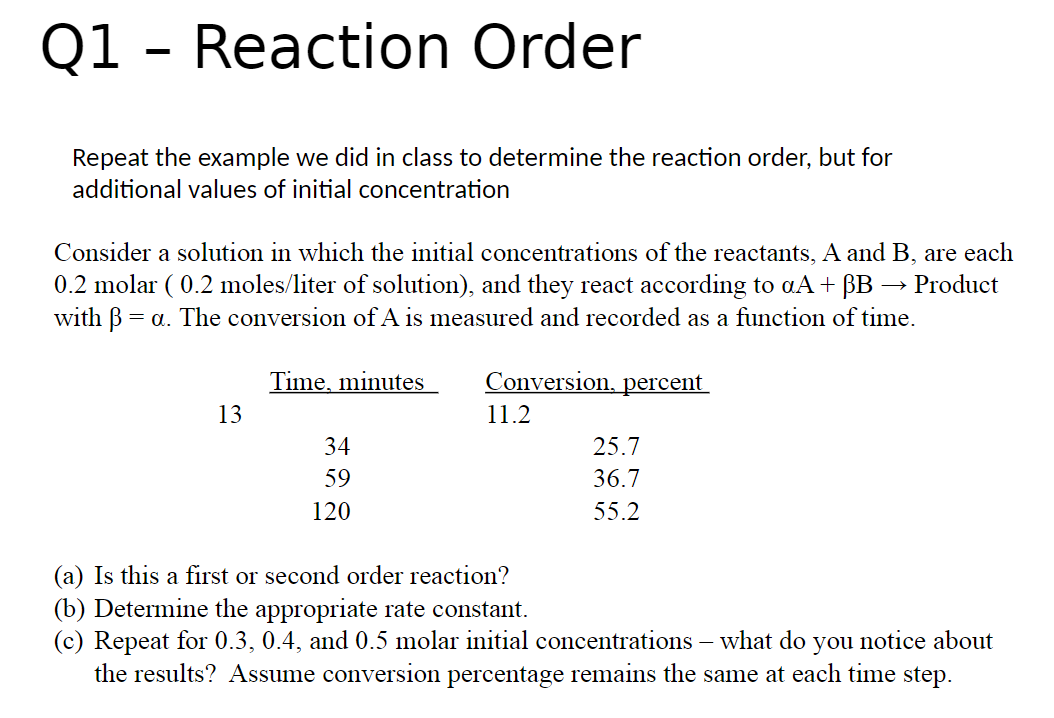
Q1 - Reaction Order Repeat the example we did in class to determine the reaction order, but for additional values of initial concentration Consider a solution in which the initial concentrations of the reactants, A and B, are each 0.2 molar ( 0.2 moles/liter of solution), and they react according to A+B Product with =. The conversion of A is measured and recorded as a function of time. (a) Is this a first or second order reaction? (b) Determine the appropriate rate constant. (c) Repeat for 0.3,0.4, and 0.5 molar initial concentrations - what do you notice about the results? Assume conversion percentage remains the same at each time step
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


