Question: q2 QUESTION 2 20 points Save Answer In an extraction process for extracting oil from soybeans, i-Pentane is used as a solvent. After the extraction
q2
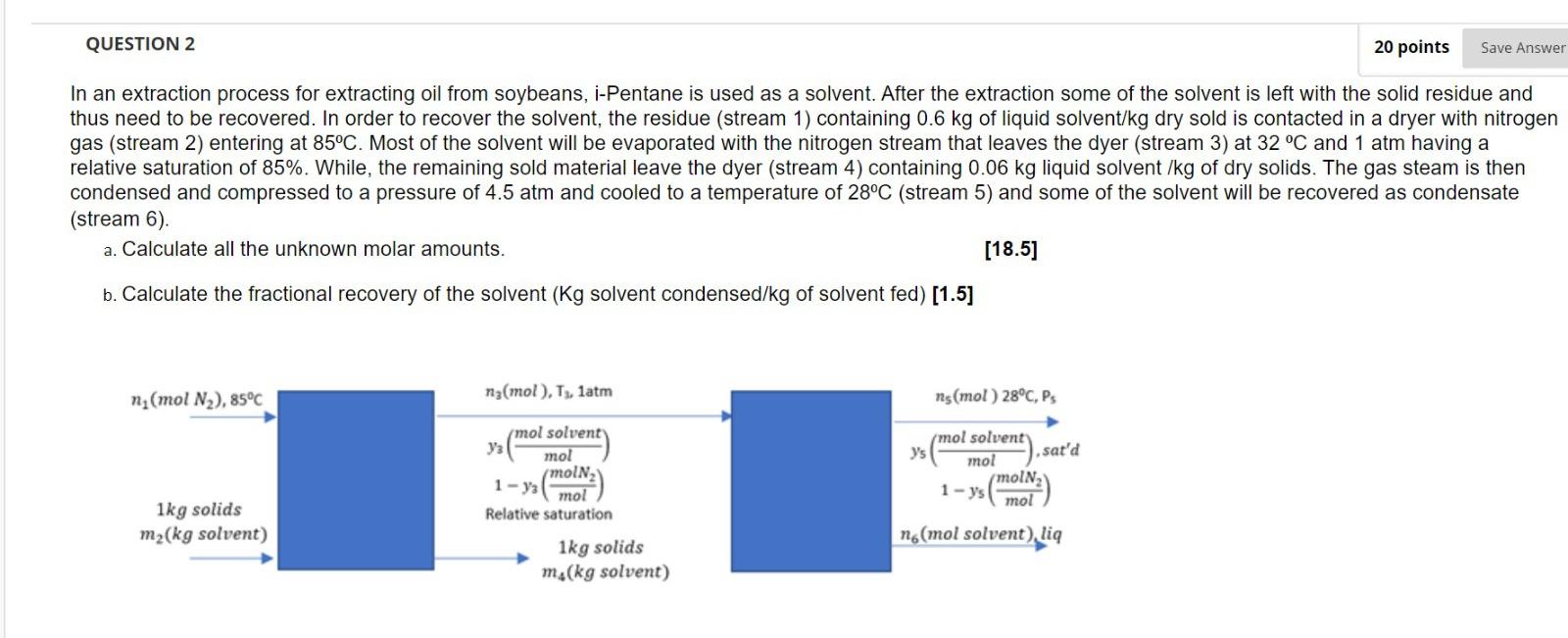
QUESTION 2 20 points Save Answer In an extraction process for extracting oil from soybeans, i-Pentane is used as a solvent. After the extraction some of the solvent is left with the solid residue and thus need to be recovered. In order to recover the solvent, the residue (stream 1) containing 0.6 kg of liquid solvent/kg dry sold is contacted in a dryer with nitrogen gas (stream 2) entering at 85C. Most of the solvent will be evaporated with the nitrogen stream that leaves the dyer (stream 3) at 32 C and 1 atm having a relative saturation of 85%. While, the remaining sold material leave the dyer (stream 4) containing 0.06 kg liquid solvent /kg of dry solids. The gas steam is then condensed and compressed to a pressure of 4.5 atm and cooled to a temperature of 28C (stream 5) and some of the solvent will be recovered as condensate (stream 6). a. Calculate all the unknown molar amounts. [18.5] b. Calculate the fractional recovery of the solvent (Kg solvent condensed/kg of solvent fed) [1.5] ni(mol N2), 85C n3(mol), TX, latm ns(mol) 28C, PS mol solvent Y3 mol mol solvent sat'd mol moln 1-ys mol 1 ->(MolN) 1kg solids m2(kg solvent) Relative saturation 1kg solids m.(kg solvent) n (mol solvent) li
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


