Question: Q2-(40p) A single-cell Solid Oxide Fuel Cell with 100cm2 active area operates at 800C. The cell consumes 3% moist hydrogen at ambient pressure. The partial
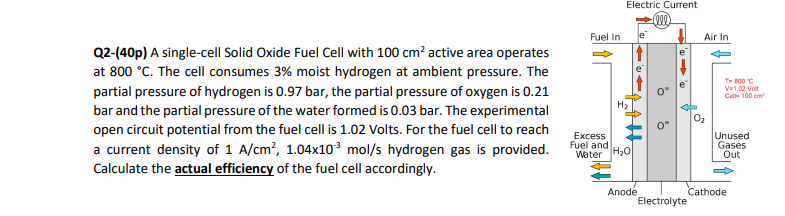
Q2-(40p) A single-cell Solid Oxide Fuel Cell with 100cm2 active area operates at 800C. The cell consumes 3% moist hydrogen at ambient pressure. The partial pressure of hydrogen is 0.97bar, the partial pressure of oxygen is 0.21 bar and the partial pressure of the water formed is 0.03 bar. The experimental open circuit potential from the fuel cell is 1.02 Volts. For the fuel cell to reach a current density of 1A/cm2,1.04103mol/s hydrogen gas is provided. Calculate the actual efficiency of the fuel cell accordingly
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


