Question: Q3. [1pts :FS20Exam3: Ch31.1B[3]]. Solute A exists on solid B surface. If this B containing A is placed in a solvent C (Ca(t=0)=O=Cao), it takes
![Q3. [1pts :FS20Exam3: Ch31.1B[3]]. Solute A exists on solid B surface.](https://dsd5zvtm8ll6.cloudfront.net/si.experts.images/questions/2024/09/66f84deba3c44_90766f84deb51044.jpg)
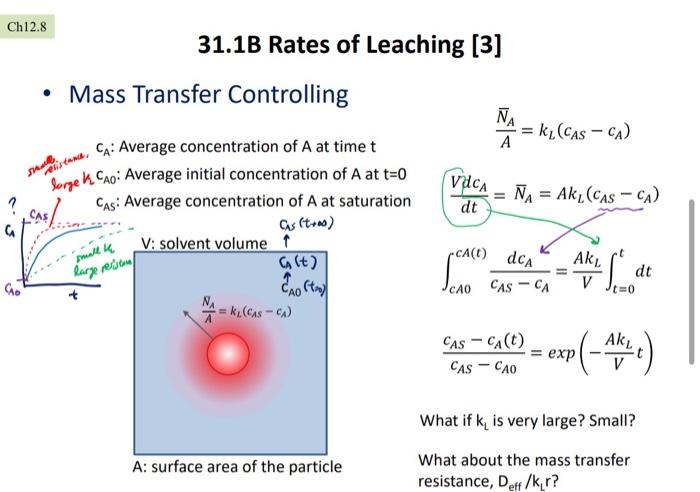
Q3. [1pts :FS20Exam3: Ch31.1B[3]]. Solute A exists on solid B surface. If this B containing A is placed in a solvent C (Ca(t=0)=O=Cao), it takes 1 hr to reach 50% of the saturation of Ain C (C.lt=1hr)=0.5CAs). Estimate the time to reach 80% of the saturation (Ca{t=?hr)=0.9CAs). Hint: Use the equation derived in Ch31.1B[3). Solve for AK.N at t=1hr first. Ch12.8 31.1B Rates of Leaching [3] Mass Transfer Controlling A = k/CAS - CA) shapes and CA: Average concentration of A at timet lorgek Cao: Average initial concentration of A at t=0 Cas: Average concentration of A at saturation Cas (tra) V: solvent volume 1 Alt) Vdca = A = Ak, (CAS-CA) dt CA() da smallia large reisen + 20 Ft) Ak V dt V Je=0 CAO CAS - CA * -ku (Casc) Ak CAS - Ca(t) CAS - CAO = exp P(-4614) What if k, is very large? Small? A: surface area of the particle What about the mass transfer resistance, Deff /kr
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


