Question: Q3 (35 p): The liquid phase reaction AP will be carried out in a 5 m volume BR operated at a constant temperature of 330
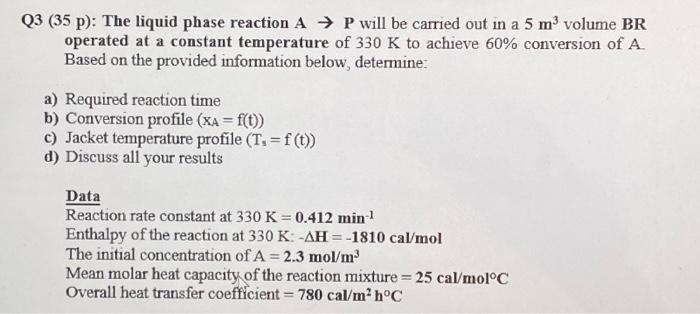
Q3 (35 p): The liquid phase reaction AP will be carried out in a 5m3 volume BR operated at a constant temperature of 330K to achieve 60% conversion of A Based on the provided information below, determine: a) Required reaction time b) Conversion profile (xA=f(t)) c) Jacket temperature profile (Ts=f(t)) d) Discuss all your results Data Reaction rate constant at 330K=0.412min1 Enthalpy of the reaction at 330K:H=1810cal/mol The initial concentration of A=2.3mol/m3 Mean molar heat capacity of the reaction mixture =25cal/molC Overall heat transfer coefficient =780cal/m2hC Q3 (35 p): The liquid phase reaction AP will be carried out in a 5m3 volume BR operated at a constant temperature of 330K to achieve 60% conversion of A Based on the provided information below, determine: a) Required reaction time b) Conversion profile (xA=f(t)) c) Jacket temperature profile (Ts=f(t)) d) Discuss all your results Data Reaction rate constant at 330K=0.412min1 Enthalpy of the reaction at 330K:H=1810cal/mol The initial concentration of A=2.3mol/m3 Mean molar heat capacity of the reaction mixture =25cal/molC Overall heat transfer coefficient =780cal/m2hC
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


