Question: Q#3, calculate mass idk why I am getting it wrong... TY for the help!! You need to create a buffer with a final pH of
Q#3, calculate mass idk why I am getting it wrong... TY for the help!!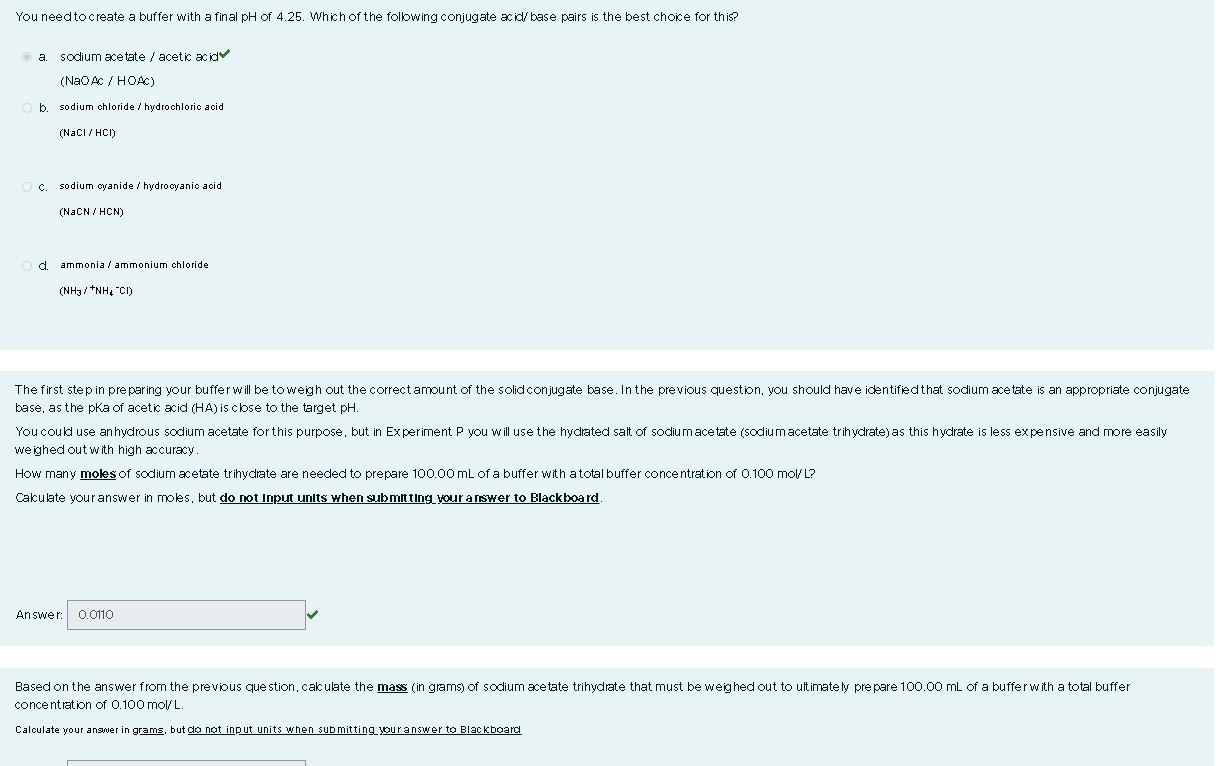
You need to create a buffer with a final pH of 4.25. Which of the folbwing conjugate acid/ base pairs is the best choice for this? a. sodium acetate / acetic acid (NaOAC/HOAC) b. sodium chloride / hydrochloric acid (NaCl/HCl) c. sodium cyanide / hydrocyanic acid (NaCN /HCN) d. ammonia/ammonium chloride (NH3i+NH4Cl) base, as the pKa of acetic acid (HA) is close to the target pH. we ighed out with high accuracy. How many molss of sodium acetate trihydrate are needed to prepare 100.00mL of a buffer with a total buffer conce ntration of 0.100 mol/ L? Calculate your answer in moles, but do not input units when submit ting your a rawer to Edackboard. Answer: concentlation of 0.100molL. Calculate your angmer in grams, but ga not input units when submitting yur answer to Blackboard
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


