Question: Q3) Given the pulse test shown below: Additional data: Second order reaction, KC20=1.2min1 Volumetric flow rate =150ga//min Time (retia) 0f(t)dt=? Determine the following a. Calculate
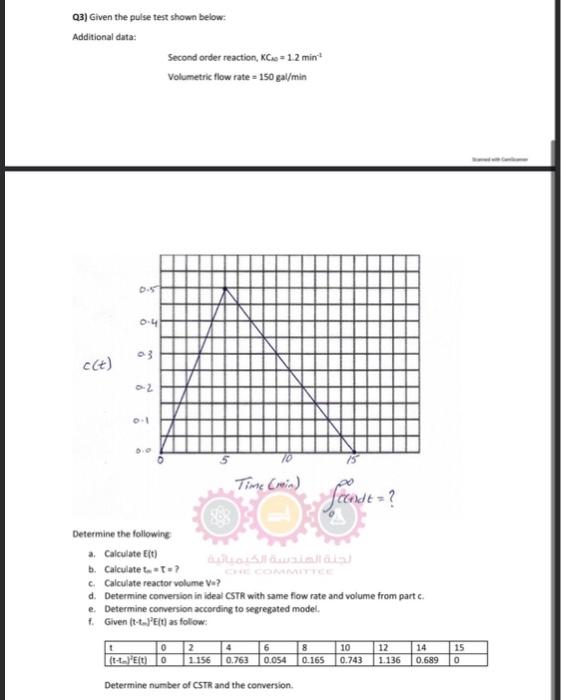
Q3) Given the pulse test shown below: Additional data: Second order reaction, KC20=1.2min1 Volumetric flow rate =150ga//min Time (retia) 0f(t)dt=? Determine the following a. Calculate E(t) b. Caiculate ti=t= ? c. Calculate reactor volume V=? d. Determine conversion in ideal CSTR with same flow rate and volume from part c. e. Determine conversion according to segregated model, f. Given (t-t-e]? E(t) as follow: Determine number of CSTR and the conversion
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


