Question: Question 1 (1 point) What does the word aqueous mean? dissolved in solvent soluble in water dissolved in water solid in water Question 2


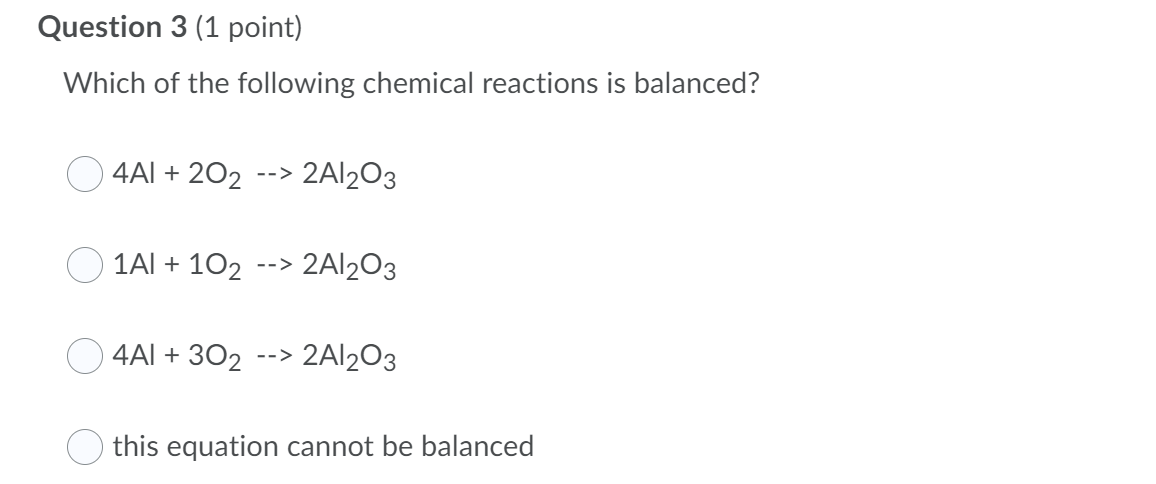
Question 1 (1 point) What does the word "aqueous" mean? dissolved in solvent soluble in water dissolved in water solid in water Question 2 (1 point) A chemical equation is balanced when the number of atoms on the left and right sides of the reaction are equal. the number of atoms on the left and right side on the reaction are not equal. the mass of one reactant is equal to one product. O nothing is equal and equilibrium is established. Question 4 (1 point) From the following list select the observations that would indicate that a chemical reaction has taken place. Select all correct answers. volume was 34.5 ml bubbles formed a solid formed my partner added red food coloring water is wet at 25 C test tubes are made of glass color changed the reaction got really hot my lab partner insists Question 3 (1 point) Which of the following chemical reactions is balanced? 4AI + 202 --> 2Al2O3 1AI + 102 --> 2Al2O3 4AI + 30 --> 2Al2O3 this equation cannot be balanced
Step by Step Solution
3.44 Rating (154 Votes )
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


