Question: Question 1 (1 point) When an element is Oxidized, which of the following are true? Electrons are Gained and the oxidation number Increases Electrons are
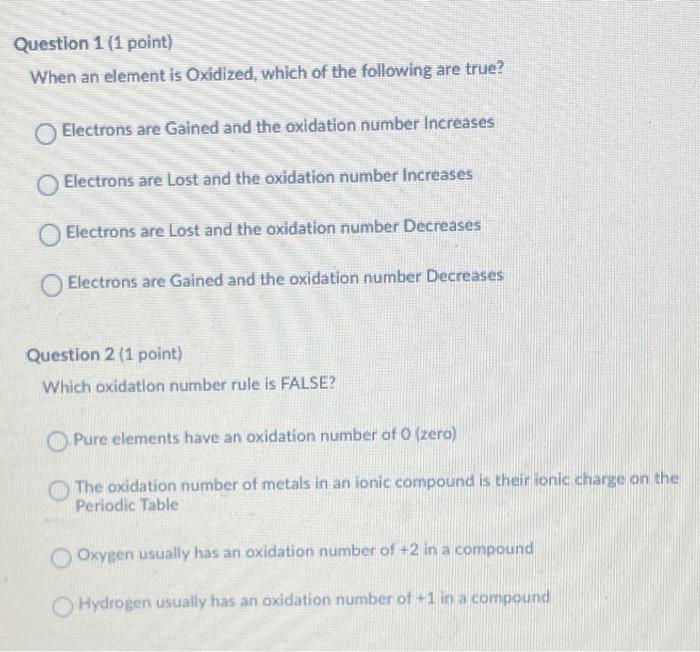
Question 1 (1 point) When an element is Oxidized, which of the following are true? Electrons are Gained and the oxidation number Increases Electrons are Lost and the oxidation number Increases Electrons are Lost and the oxidation number Decreases Electrons are Gained and the oxidation number Decreases Question 2 (1 point) Which oxidation number rule is FALSE? Pure elements have an oxidation number of O (zero) The oxidation number of metals in an ionic compound is their lonic charge on the Periodic Table Oxygen usually has an oxidation number of +2 in a compound Hydrogen usually has an oxidation number of +1 in a compound
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


