Question: QUESTION 1 (20 marks) Table 1 shows the experimental data used to determine the reactor volume of the reaction, A + B + C. The
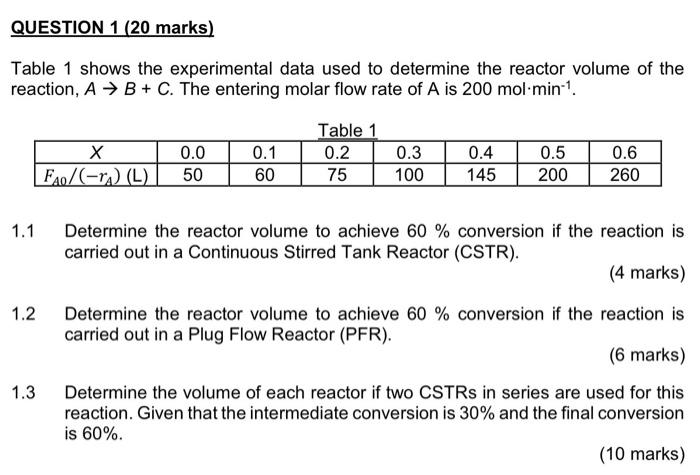
QUESTION 1 (20 marks) Table 1 shows the experimental data used to determine the reactor volume of the reaction, A + B + C. The entering molar flow rate of A is 200 mol min-1 F20/(-r.) (L) 0.0 50 0.1 60 Table 1 0.2 75 0.3 100 0.4 145 0.5 200 0.6 260 1.1 Determine the reactor volume to achieve 60% conversion if the reaction is carried out in a Continuous Stirred Tank Reactor (CSTR). (4 marks) 1.2 1.3 Determine the reactor volume to achieve 60 % conversion if the reaction is carried out in a Plug Flow Reactor (PER). (6 marks) Determine the volume of each reactor if two CSTRs in series are used for this reaction. Given that the intermediate conversion is 30% and the final conversion is 60%. (10 marks)
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


