Question: Question 1 (25 marks) Dinitrogen pentoxide decomposes into nitrogen dioxide and molecular oxygen. (a) Write a balanced chemical equation for the decomposition. (3 marks) (b)
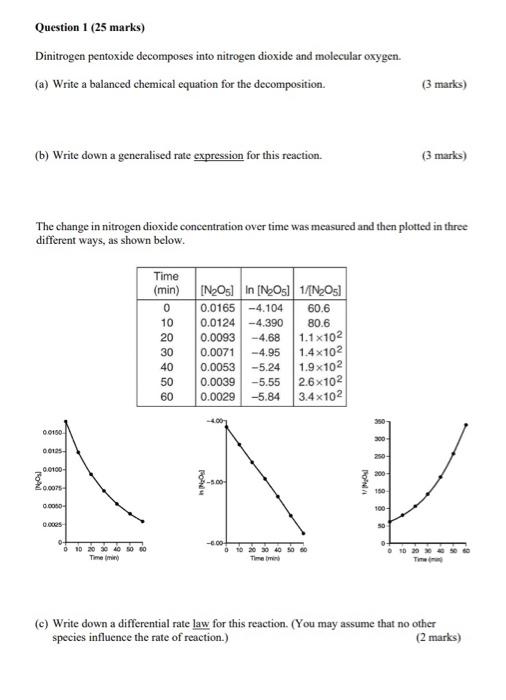

Question 1 (25 marks) Dinitrogen pentoxide decomposes into nitrogen dioxide and molecular oxygen. (a) Write a balanced chemical equation for the decomposition. (3 marks) (b) Write down a generalised rate expression for this reaction. (3 marks) The change in nitrogen dioxide concentration over time was measured and then plotted in three different ways, as shown below. (c) Write down a differential rate law for this reaction. (You may assume that no other species influence the rate of reaction.) (2 marks) (d) Using the data in the table in (b), determine the rate coefficient, k, for the reaction. (5 marks) (e) Calculate the half-life of this reaction, in minutes. (3 marks) (f) Describe what the following terms mean. (9 marks) (i) Half-life: (ii) Complex reaction
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


