Question: Question 1 ( a ) A ( 0 . 2 mathrm { ~m } ^ { 3 } ) tank initially contains
Question
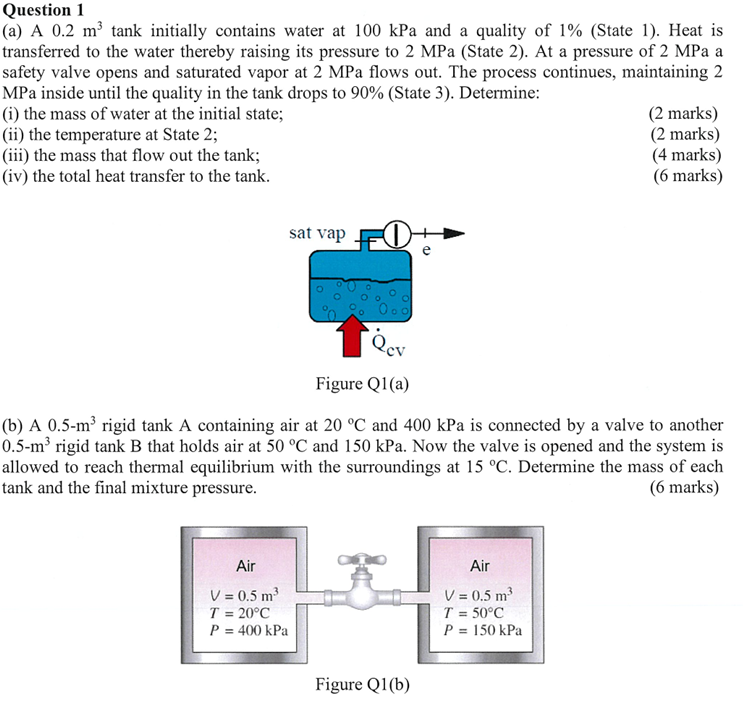
a A mathrm~m tank initially contains water at kPa and a quality of State Heat is transferred to the water thereby raising its pressure to MPa State At a pressure of MPa a safety valve opens and saturated vapor at MPa flows out. The process continues, maintaining MPa inside until the quality in the tank drops to State Determine:
i the mass of water at the initial state;
ii the temperature at State ;
iii the mass that flow out the tank;
iv the total heat transfer to the tank.
marks
marks
marks
marks
b A mathrmm rigid tank A containing air at circmathrmC and kPa is connected by a valve to another mathrmm rigid tank B that holds air at circmathrmC and kPa Now the valve is opened and the system is allowed to reach thermal equilibrium with the surroundings at circmathrmC Determine the mass of each tank and the final mixture pressure.
marks
Step by Step Solution
There are 3 Steps involved in it
1 Expert Approved Answer
Step: 1 Unlock


Question Has Been Solved by an Expert!
Get step-by-step solutions from verified subject matter experts
Step: 2 Unlock
Step: 3 Unlock


