Question: Question 1 Oo A 15 mol% H2SO4 solution that contains 10.00 moles of H20 contains ......... of solution 00 Question 2 . H2S04 mass fraction
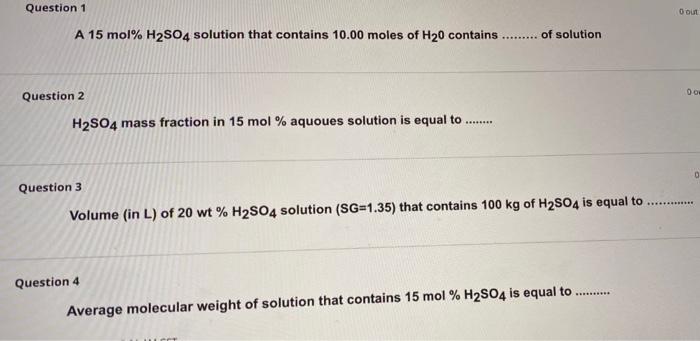
Question 1 Oo A 15 mol% H2SO4 solution that contains 10.00 moles of H20 contains ......... of solution 00 Question 2 . H2S04 mass fraction in 15 mol % aquoues solution is equal to 0 Question 3 Volume (in L) of 20 wt% H2SO4 solution (SG=1.35) that contains 100 kg of H2SO4 is equal to Question 4 Average molecular weight of solution that contains 15 mol % H2SO4 is equal to
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


