Question: QUESTION 1 (PO1, C01, C2) 1. A chemist performs an experiment to find the acidity of magnesium hydroxide solution in hydrochloric acid. He obtains the
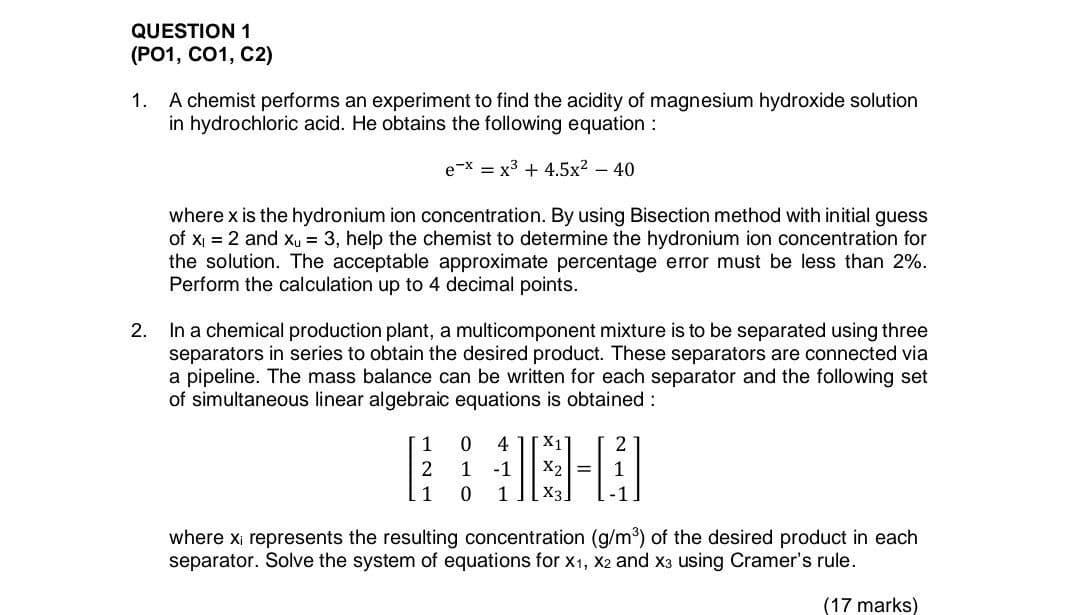
QUESTION 1 (PO1, C01, C2) 1. A chemist performs an experiment to find the acidity of magnesium hydroxide solution in hydrochloric acid. He obtains the following equation : e-x = x3 + 4.5x2 - 40 where x is the hydronium ion concentration. By using Bisection method with initial guess of Xi = 2 and Xu = 3, help the chemist to determine the hydronium ion concentration for the solution. The acceptable approximate percentage error must be less than 2%. Perform the calculation up to 4 decimal points. 2. In a chemical production plant, a multicomponent mixture is to be separated using three separators in series to obtain the desired product. These separators are connected via a pipeline. The mass balance can be written for each separator and the following set of simultaneous linear algebraic equations is obtained : 1 : 1-2 1 2 1 0 1 0 4 -1 1 X1 X2= X3 where xi represents the resulting concentration (g/m) of the desired product in each separator. Solve the system of equations for X1, X2 and X3 using Cramer's rule. (17 marks)
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


