Question: Question 1. The following data were obtained for the initial rates of the balanced reaction: 3H2SO4+2NO2HNO3+3SO2+2H2O a) Determine the reaction order for both reactants and
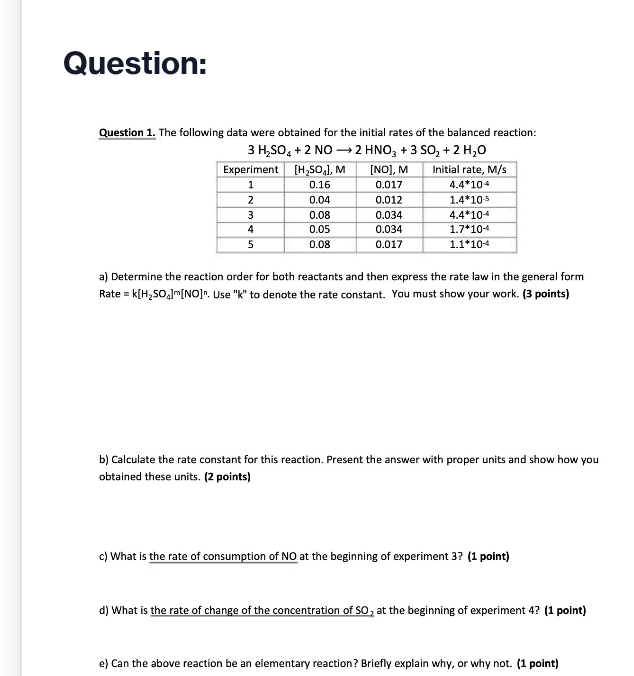
Question 1. The following data were obtained for the initial rates of the balanced reaction: 3H2SO4+2NO2HNO3+3SO2+2H2O a) Determine the reaction order for both reactants and then express the rate law in the general form Rate =k[H2SO4]m[NO]n. Use "k" to denote the rate constant. You must show your work. (3 points) b) Calculate the rate constant for this reaction. Present the answer with proper units and show how you obtained these units. (2 points) c) What is the rate of consumption of NO at the beginning of experiment 3? (1 point) d) What is the rate of change of the concentration of SO2 at the beginning of experiment 4 ? (1 point) e) Can the above reaction be an elementary reaction? Briefly explain why, or why not. (1 point)
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


