Question: Question 1: Unit operations a. As a chemical engineer, you have a desire to purify a variety of vitamins to be used as food supplements.

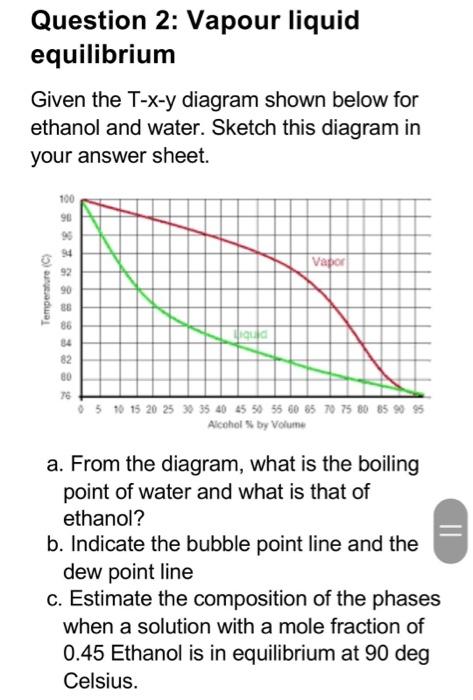

Question 1: Unit operations a. As a chemical engineer, you have a desire to purify a variety of vitamins to be used as food supplements. How can you use diffusion to characterize the size of the vitamins in solution? b. Ammonia, the major material for fertilizer, is made by reacting nitrogen and hydrogen under pressure. The product gas can be washed with water to dissolve the ammonia and separate it from other unreacted gases. How can you correlate the dissolution rate of ammonia during washing? Question 2: Vapour liquid equilibrium Given the T-x-y diagram shown below for ethanol and water. Sketch this diagram in your answer sheet. 100 90 95 Vapor Temperature (C) 92 90 88 86 34 82 80 ligula 0 5 10 15 20 25 35 40 45 50 55 60 65 70 75 80 85 95 95 Alcohol by Volume a. From the diagram, what is the boiling point of water and what is that of ethanol? b. Indicate the bubble point line and the dew point line c. Estimate the composition of the phases when a solution with a mole fraction of 0.45 Ethanol is in equilibrium at 90 deg Celsius. Qustion 3: Distillation A liquor of 0.30 mole fraction of benzene and the rest toluene is fed to a continuous still to give a top product of 0.90 mole fraction benzene and a bottom product of 0.95 mole fraction toluene. If the reflux ratio is 5.0, how many plates are required (a) if the feed is saturated vapour? (b) if the feed is liquid at 283 K
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


