Question: Question #1: (using charles's law) Question #2: (Solving applications of Boyle's Law) Question #3: (Interconverting pressure and force) AND SOLIDS E Using Charles's Law An
Question #1: (using charles's law)
Question #2: (Solving applications of Boyle's Law)
 Question #3: (Interconverting pressure and force)
Question #3: (Interconverting pressure and force)
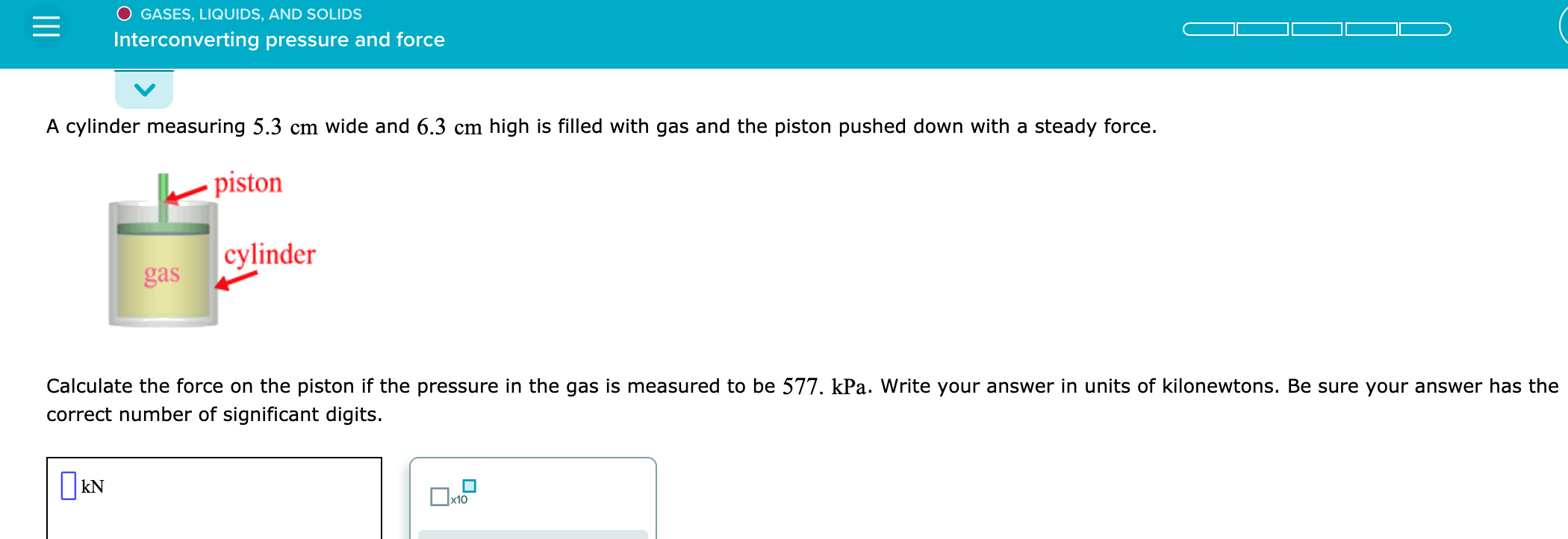
AND SOLIDS E Using Charles's Law An arctic weather balloon is filled with 7.13 L of helium gas inside a prep shed. The temperature inside the shed is 11. C. The balloon is then taken outside, where the temperature is 30. C. Calculate the new volume of the balloon. You may assume the pressure on the balloon stays constant at exactly 1 atm. Be sure your answer has the correct number of significant digits. OL x10 s ? = GASES, LIQUIDS, AND SOLIDS Solving applications of Boyle's Law A gas storage cylinder in an ordinary chemical laboratory measures 4.8 cm wide and 19. cm high. This is the label on it. Contents: N2 gas Pressure: 16.8 atm If the cylinder is opened and the gas allowed to escape into a large empty plastic bag, what will be the final volume of nitrogen gas, including what's collected in the plastic bag and what's left over in the cylinder? Write your answer in liters. Be sure your answer has the correct number of significant digits. L Dx10 O GASES, LIQUIDS, AND SOLIDS Interconverting pressure and force A cylinder measuring 5.3 cm wide and 6.3 cm high is filled with gas and the piston pushed down with a steady force. piston cylinder gas Calculate the force on the piston if the pressure in the gas is measured to be 577. kPa. Write your answer in units of kilonewtons. Be sure your answer has the correct number of significant digits. k
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


