Question: Question 1: Which will be stopped by a 1-cm-thick layer of steel? A) Alpha radiation B) Beta radiation C) Gamma radiation D) Both alpha


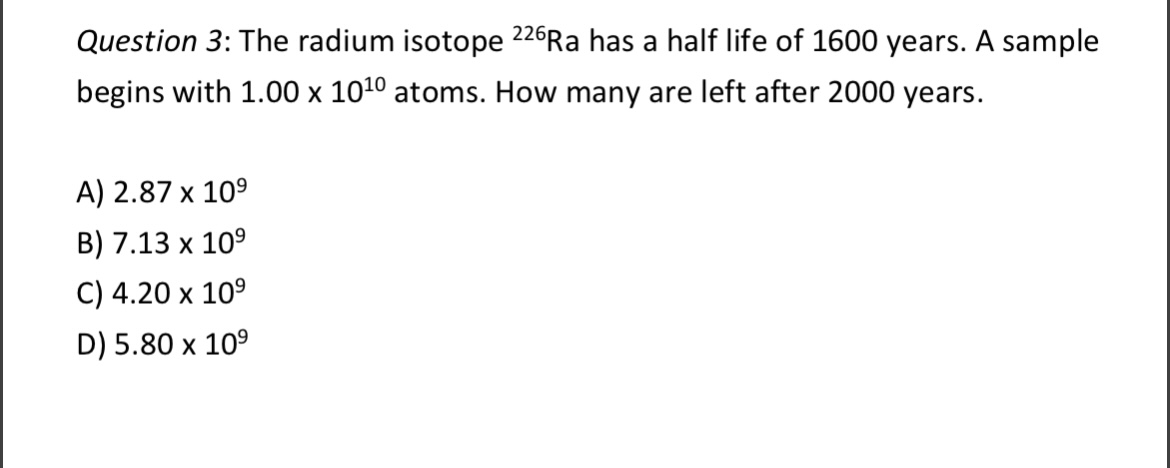

Question 1: Which will be stopped by a 1-cm-thick layer of steel? A) Alpha radiation B) Beta radiation C) Gamma radiation D) Both alpha and beta radiation Question 2: Is 238Pu(Z = 84) 236 U(Z = 82) + a possible decay mode? Alpha radiation is fast moving 4He nuclei. A) Yes B) No C) Need to know the energy of the emitted alpha particle. Question 3: The radium isotope 226 Ra has a half life of 1600 years. A sample begins with 1.00 x 1010 atoms. How many are left after 2000 years. A) 2.87 x 109 B) 7.13 x 109 C) 4.20 x 10 D) 5.80 x 10 Question 4: 100 g of a radioactive element X are placed in a sealed box. The mass number is 137. Let the half-life of this isotope be 2 days. After 4 days have passed, what the number of radioactive atoms X left in the box? A) 1.1 x 1023 B) 2.2 x 1023 C) 1.5 x 1025 D) 2.5 x 10-3
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


