Question: QUESTION 1: Why would adding concentrated H2SO4 in the last step of the reaction help the crystallization process if solid product does not initially appear?
QUESTION 1: Why would adding concentrated H2SO4 in the last step of the reaction help the crystallization process if solid product does not initially appear?
QUESTION 2: What is the gas trapped in the gas trap during the reaction?

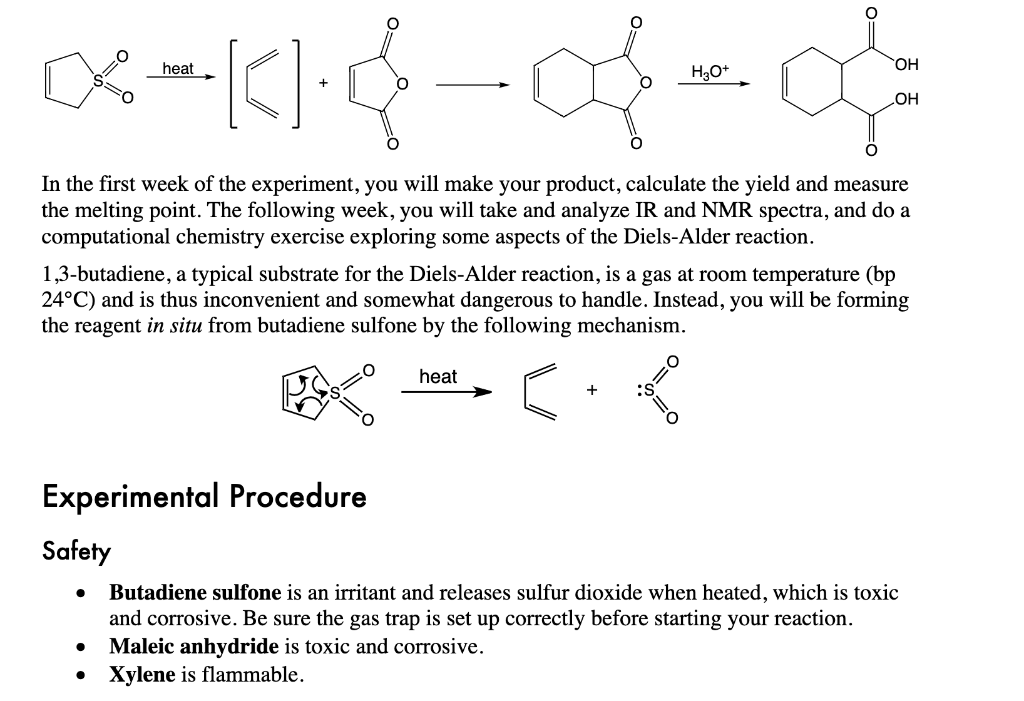
Why would adding concentrated H2SO4 in the last step of the reaction help the crystallization process if solid product does not initially appear? What is the gas "trapped" in the gas trap during the reaction? H3O+ In the first week of the experiment, you will make your product, calculate the yield and measure the melting point. The following week, you will take and analyze IR and NMR spectra, and do a computational chemistry exercise exploring some aspects of the Diels-Alder reaction. 1,3-butadiene, a typical substrate for the Diels-Alder reaction, is a gas at room temperature (bp 24C ) and is thus inconvenient and somewhat dangerous to handle. Instead, you will be forming the reagent in situ from butadiene sulfone by the following mechanism. Experimental Procedure Safety - Butadiene sulfone is an irritant and releases sulfur dioxide when heated, which is toxic and corrosive. Be sure the gas trap is set up correctly before starting your reaction. - Maleic anhydride is toxic and corrosive. - Xylene is flammable
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


