Question: Question 14 (based on material in Units 16 and 18) (a) Liquid nitrogen has a density of 807kgm 2 at its boiling point of 7TK.
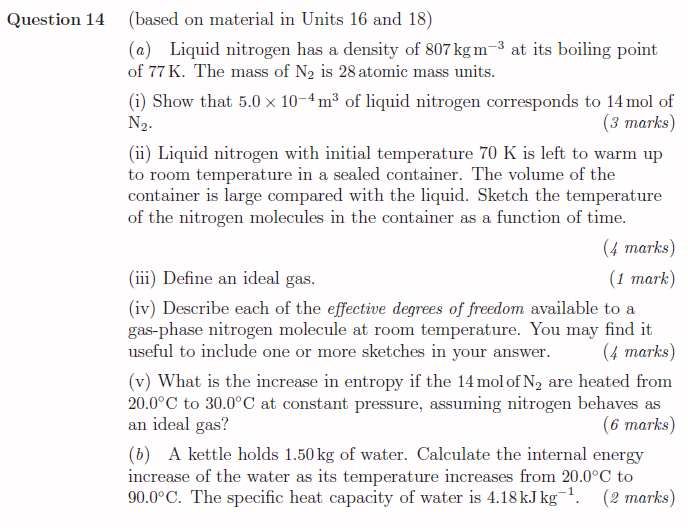
Question 14 (based on material in Units 16 and 18) (a) Liquid nitrogen has a density of 807kgm 2 at its boiling point of 7TK. The mass of N 1s 28 atomic mass units. i) Show that 5.0 x 10~*m? of liquid nitrogen corresponds to 14 mol of q g p Na. (3 marks) (1) Liquid nitrogen with initial temperature 70 K is left to warm up to room temperature in a sealed container. The volume of the container is large compared with the liqgmid. Sketch the temperature of the nitrogen molecules in the container as a function of time. (4 marks) (n1) Define an ideal gas. (1 mark) (1v) Describe each of the effective degrees of freedom available to a gas-phase nitrogen molecule at room temperature. You may find it useful to include one or more sketches in your answer. (4 marks) (v) What is the increase in entropy if the 14 molof N, are heated from 20.0C to 30.0C at constant pressure, assuming nitrogen behaves as an ideal gas? (6 marks) (6) A kettle holds 1.50kg of water. Calculate the internal energy mcrease of the water as its temperature increases from 20.0C to 00.0C. The specific heat capacity of water is 4.18kJ kg '. (2 marks)
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


