Question: Question 17 For the given reaction: 2 Na2O2 (s) + 2 H20 (1) 4 NaOH (s) + O2 (g) AH =-126 kJ/mol. a) Calculate the
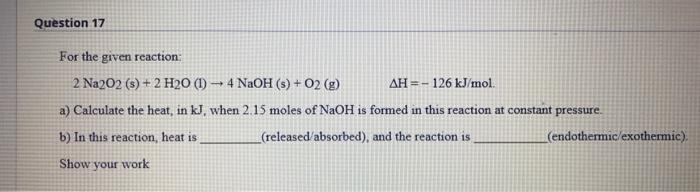
Question 17 For the given reaction: 2 Na2O2 (s) + 2 H20 (1) 4 NaOH (s) + O2 (g) AH =-126 kJ/mol. a) Calculate the heat, in kJ, when 2.15 moles of NaOH is formed in this reaction at constant pressure. b) In this reaction, heat is _(released absorbed), and the reaction is (endothermic/exothermic) Show your work
Step by Step Solution
There are 3 Steps involved in it
1 Expert Approved Answer
Step: 1 Unlock


Question Has Been Solved by an Expert!
Get step-by-step solutions from verified subject matter experts
Step: 2 Unlock
Step: 3 Unlock


