Question: Question 18 1 Point The freezing points and boiling points of the solution which contains 22.1g NaCl in 129 mL of water is and respectively

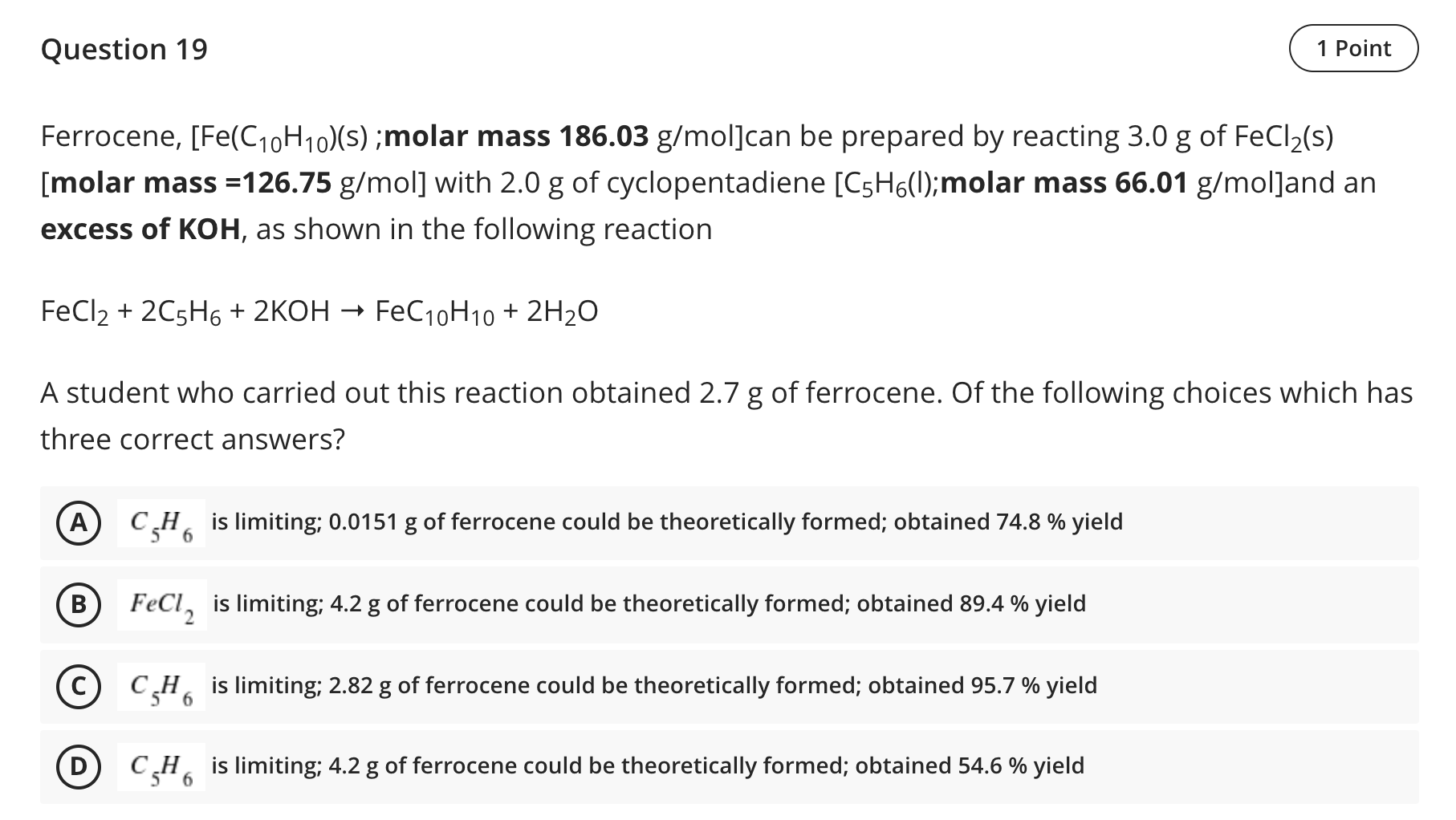
Question 18 1 Point The freezing points and boiling points of the solution which contains 22.1g NaCl in 129 mL of water is and respectively [kf for water= 1.86C/m; kb for water= 0.52 C/m; density of water = 1 g/mL] A 10.90C; 103.05C B - 10.90C : 103.05C - 1.59C ; 100.5C D -1.90C ; 103.05C Question 19 1 Point Ferrocene, [Fe(C10H10)(s);molar mass 186.03 g/moljcan be prepared by reacting 3.0 g of FeCl2(s) [molar mass =126.75 g/mol] with 2.0 g of cyclopentadiene [C5H6(l); molar mass 66.01 g/mol]and an excess of KOH, as shown in the following reaction FeCl2 + 2C5H6 + 2KOH FeC10H10 + 2H2O A student who carried out this reaction obtained 2.7 g of ferrocene. Of the following choices which has three correct answers? A C,He is limiting; 0.0151 g of ferrocene could be theoretically formed; obtained 74.8 % yield 6 B FeCl, is limiting; 4.2 g of ferrocene could be theoretically formed; obtained 89.4 % yield CH is limiting; 2.82 g of ferrocene could be theoretically formed; obtained 95.7 % yield D CH6 is limiting; 4.2 g of ferrocene could be theoretically formed; obtained 54.6 % yield 56
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


