Question: Question 3 1 Point The freezing points and boiling points of the solution which contains 25.0 g NaCl in 129 mL of water is and
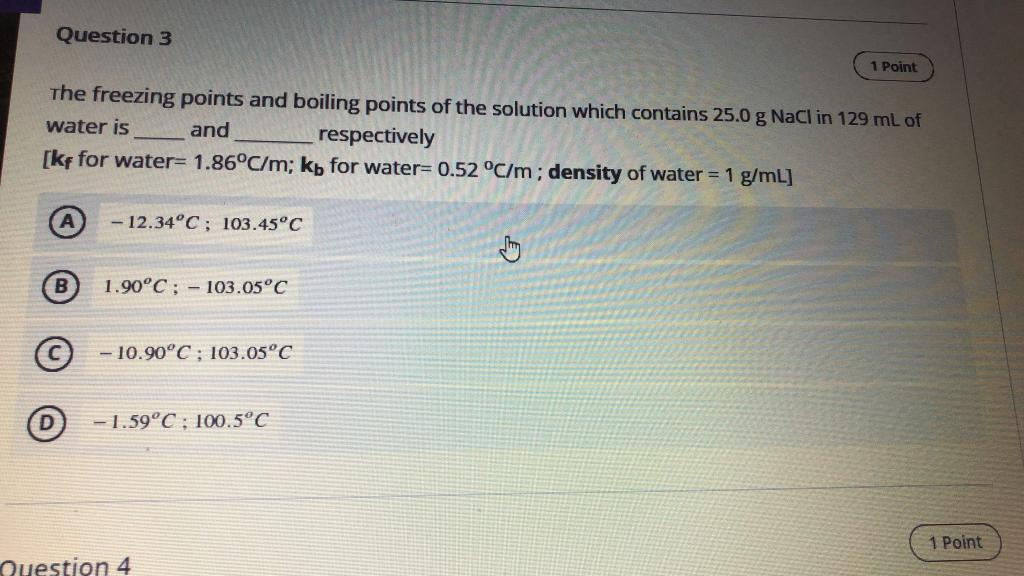
Question 3 1 Point The freezing points and boiling points of the solution which contains 25.0 g NaCl in 129 mL of water is and respectively [kf for water= 1.86C/m; kb for water=0.52 C/m; density of water = 1 g/mL] - 12.34C; 103.45C 1.90C : - 103.05C - 10.90C ; 103.05C 0 - 1.59C ; 100.5C 1 Point Question 4
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


